


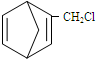
 £¬ŗ¬¹²éī½į¹¹µÄŌĮĻæÉŅŌŹĒ
£¬ŗ¬¹²éī½į¹¹µÄŌĮĻæÉŅŌŹĒ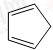 »ņ
»ņ £®£ØŠ“½į¹¹¼ņŹ½£©
£®£ØŠ“½į¹¹¼ņŹ½£© µÄŗĻ³ÉĀ·ĻߣØĘäĖūĪŽ»śŹŌ¼ĮČĪŃ”£©£®
µÄŗĻ³ÉĀ·ĻߣØĘäĖūĪŽ»śŹŌ¼ĮČĪŃ”£©£®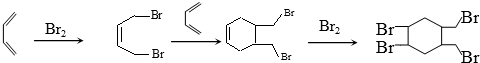 £®
£® ·ÖĪö £Ø1£©¾Ż¹²éī½į¹¹µÄŗ¬Ņå·ÖĪö£»
£Ø2£©ĢģČ»Ļš½ŗŹĒŅģĪģ¶žĻ©£¬½į¹¹¼ņŹ½ĪŖ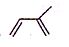 £¬»ÆѧĆū³ĘŹĒ2-¼×»ł-1£¬3-¶”¶žĻ©£¬¾Ż“Ė·ÖĪö£»
£¬»ÆѧĆū³ĘŹĒ2-¼×»ł-1£¬3-¶”¶žĻ©£¬¾Ż“Ė·ÖĪö£»
£Ø3£©ŅŖÖʱø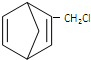 £¬ŠčŅŖŗ¬ÓŠ¹²éī½į¹¹µÄ5ŌŖ»·£»
£¬ŠčŅŖŗ¬ÓŠ¹²éī½į¹¹µÄ5ŌŖ»·£»
£Ø4£©“Ó1£¬3-¶”¶žĻ©ŗĻ³É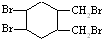 £¬æÉŅŌĻČČĆ1£¬3-¶”¶žĻ©Óėäå1£ŗ1¼Ó³É£¬Éś³É1£¬4-¶žäå-2-¶”Ļ©£¬ŌŁÓė1£¬3-¶”¶žĻ©·“Ó¦µĆµ½6ŌŖ»·£¬Óėäå¼Ó³ÉÖĘµĆ£®
£¬æÉŅŌĻČČĆ1£¬3-¶”¶žĻ©Óėäå1£ŗ1¼Ó³É£¬Éś³É1£¬4-¶žäå-2-¶”Ļ©£¬ŌŁÓė1£¬3-¶”¶žĻ©·“Ó¦µĆµ½6ŌŖ»·£¬Óėäå¼Ó³ÉÖĘµĆ£®
½ā“š ½ā£ŗ£Ø1£©·Ö×ÓÖŠ“ęŌŚµ„Ė«¼ü½»Ģę³öĻֵĽį¹¹³ĘĪŖ¹²éī½į¹¹£¬·ūŗĻ¹²éī½į¹¹µÄĪŖbd£¬¹Ź“š°øĪŖ£ŗbd£»
£Ø2£©ĢģČ»Ļš½ŗŹĒŅģĪģ¶žĻ©£¬½į¹¹¼ņŹ½ĪŖ £¬ĘäÓėäå¼Ó³É²śĪļæÉÄÜĪŖ£ŗCH2BrCHBrC£ØCH3£©=CH2£¬CH2=CHC£ØCH3£©BrCH2Br£¬CH2BrCH=C£ØCH3£©CH2Br£¬CH2BrCHBrC£ØCH3£©BrCH2Br£¬¹²4ÖÖ£¬¹Ź“š°øĪŖ£ŗ4£»
£¬ĘäÓėäå¼Ó³É²śĪļæÉÄÜĪŖ£ŗCH2BrCHBrC£ØCH3£©=CH2£¬CH2=CHC£ØCH3£©BrCH2Br£¬CH2BrCH=C£ØCH3£©CH2Br£¬CH2BrCHBrC£ØCH3£©BrCH2Br£¬¹²4ÖÖ£¬¹Ź“š°øĪŖ£ŗ4£»
£Ø3£©ÓĆ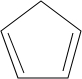 Óė
Óė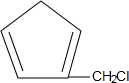 æÉŅŌÖʵĆ
æÉŅŌÖʵĆ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ »ņ
»ņ £»
£»
£Ø4£©“Ó1£¬3-¶”¶žĻ©ŗĻ³É µÄŗĻ³ÉĀ·ĻßĪŖ
µÄŗĻ³ÉĀ·ĻßĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļĪļµÄŗĻ³É£¬¹²éīĻ©ĢžµÄŠŌÖŹ£¬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņā°ŃĪÕÓŠ»śĪļ¹ŁÄÜĶŵıä»ÆŅŌ¼°·“Ó¦Ģõ¼ž£®


 ÖĒȤŗ®¼Ł×÷ŅµŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø
ÖĒȤŗ®¼Ł×÷ŅµŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÄĘČÜÓŚĖ®µÄ·“Ó¦£ŗ2Na+2H2OØT2NaOH+H2”ü | |
| B£® | Ģ¼ĖįÄĘČÜŅŗŗĶĻ”“×Ėį·“Ó¦£ŗCO2-3+2H+ØTH2O+CO2”ü | |
| C£® | ĻņBa£ØOH£©2ČÜŅŗÖŠÖšµĪ¼ÓČėĮņĖįĒāÄĘČÜŅŗÖĮÖŠŠŌ£ŗ2H++SO2-4+Ba2++2OH-ØTBaSO4”ż+2H2O | |
| D£® | Įņ»ÆŃĒĢśÖŠ¼ÓČėĻ”ĻõĖį£ŗFeS+2H+ØTFe2++H2S”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČĪļÖŹµÄĮæµÄĮņÕōĘųŗĶĮņ¹ĢĢå·Ö±šĶźČ«Č¼ÉÕ£¬ŗóÕ߷ųöµÄČČĮæ¶ą | |
| B£® | Óɵ„ÖŹA×Ŗ»ÆĪŖµ„ÖŹB”÷H=+119kJ/mol£¬æÉÖŖµ„ÖŹB±Čµ„ÖŹAĪČ¶Ø | |
| C£® | Ļ”ČÜŅŗÖŠ£ŗH+£Øaq£©+OH-£Øaq£©=H2O£Øl£©”÷H=-57.3kJ/mol | |
| D£® | ŌŚ25”ę”¢101kPaŹ±£¬2g H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö285.8kJČČĮ棬Ōņ±ķŹ¾H2Č¼ÉÕČȵĻÆѧ·½³ĢŹ½ĪŖ2H2£Øg£©+O2£Øg£©=2H2O£Øl£©”÷H=-571.6kJ/mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĆܱÕČŻĘ÷ÖŠ2molNOÓė1molO2³ä·Ö·“Ó¦ŗó£¬ČŻĘ÷ÄŚĘųĢåµÄ·Ö×ÓŹżĪŖ2NA | |
| B£® | ÓƶčŠŌµē¼«µē½āCuSO4ČÜŅŗŗó£¬Čē¹ū¼ÓČė0.1molCu£ØOH£©2ÄÜŹ¹ČÜŅŗø“Ō£¬ŌņµēĀ·ÖŠ×ŖŅʵē×ӵďżÄæĪŖ0.2NA | |
| C£® | 142 g Na2SO4ŗĶNa2HPO4¹ĢĢå»ģŗĻĪļÖŠ£¬ŅõŃōĄė×Ó×ÜŹżĪŖ3NA | |
| D£® | ¹żŃõ»ÆÄĘÓėĖ®·“Ó¦Ź±£¬Éś³É0.1molŃõĘų×ŖŅʵĵē×ÓŹżĪŖ0.4NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ½šŹōīŃŠŌÄÜÓÅŌ½£¬±»ÓžĪŖ¼ĢFe”¢AlŗóÓ¦ÓĆ¹ć·ŗµÄ”°µŚČż½šŹō”±£®
½šŹōīŃŠŌÄÜÓÅŌ½£¬±»ÓžĪŖ¼ĢFe”¢AlŗóÓ¦ÓĆ¹ć·ŗµÄ”°µŚČż½šŹō”±£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×Ėį | B£® | ŅŅ¶žĖį | C£® | ±ūĖį | D£® | ¶”Ėį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | VB2µē¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ£ŗ2VB2+11H2O-22e-ØTV2O5+2B2O3+22H+ | |
| B£® | ĶāµēĀ·ÖŠµē×ÓÓÉcµē¼«Į÷ĻņVB2µē¼« | |
| C£® | µē½ā¹ż³ĢÖŠ£¬cµē¼«±ķĆęĻČÓŠŗģÉ«ĪļÖŹĪö³ö£¬ŗóÓŠĘųÅŻ²śÉś | |
| D£® | ČōB×°ÖĆÄŚµÄŅŗĢåĢå»żĪŖ100 mL£¬ŌņCuSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ0.05 mol/L |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com