
��֪��Ӧ2CH3OH(g) CH3OCH3(g)+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/mol��L-1 | 0.44 | 0.6 | 0.6 |
������������ȷ���� ( )
A���÷�Ӧ��ƽ�ⳣ������ʽΪK=[c(CH3OCH3)��c(H2O)]/c(CH3OH)
B����ʱ�����淴Ӧ���ʵĴ�С��v����v��
C������10 min��Ӧ�ﵽƽ�⣬��ʱc(CH3OH)=0.04 mol��L����
D��0��10min��ƽ����Ӧ����v(CH3OH)=1.6 mol��(L��min)����
 ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ� ��
A�����ؽᾧ�������ᴿ�����ᣨ��ɰ�ӣ�������أ����Ȼ��ƣ�������Ϊ����
B���Ӻ�������ȡ�⣬�辭������ʵ�鲽�裺���ա��ܽ⡢���ˡ���ȡ��Һ������
C�����ȷ������Է���I2��NH4Cl��������
D��������KMnO4��Һ���Լ����̷���FeSO4��7H2O���Ƿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017��������������һ��ʵ����һ12�¿���ѧ���������棩 ���ͣ�ѡ����
����������Ͷ�뵽�Ȼ�������Һ�У��ɹ۲쵽�������ǣ� ��
A.�������ɰ�ɫ���� B.�������ɺ��ɫ����
C.���ճ��ֻ���ɫ���� D.�ޱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ������
(1)���÷�ӦCu��2FeCl3=CuCl2��2FeCl2��Ƴ���ͼ��ʾԭ��أ��ش��������⣺

��д���缫��Ӧʽ������___________________������_________________��
��ͼ��X��Һ��________��Y��Һ��________��
��ԭ��ع���ʱ�������е�________(�����������)������X��Һ�����ƶ���
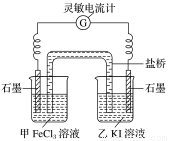
(2)�����ʺϵ�����������Ӧ2Fe3����2I�� 2Fe2����I2����Ƴ���ͼ��ʾ��ԭ��ء�
2Fe2����I2����Ƴ���ͼ��ʾ��ԭ��ء�
��ش��������⣺
��Ӧ��ʼʱ������ʯī�缫�Ϸ���____________(���������ԭ��)��Ӧ���缫��ӦʽΪ________________������ʯī�缫�Ϸ���______________��Ӧ���缫��ӦʽΪ___________��
�ڵ����ƶ���Ϊ0ʱ����Ӧ�ﵽƽ��״̬����ʱ�ڼ��м���FeCl2���壬�����е�ʯī��___________(���������)�����õ缫�ĵ缫��ӦʽΪ____________________��
(3)���÷�Ӧ2Cu��O2��2H2SO4=2CuSO4��2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪���ᡢ�������������Һ�д�������ƽ�⼰���Ӧ��ƽ�ⳣ��
CH3COOH CH3COO����H�� ��H1��0 (K1��1.75��10��5)
CH3COO����H�� ��H1��0 (K1��1.75��10��5)
CH3COO����H2O CH3COOH��OH�� ��H2��0 ( K2��5.71��10��10 )
CH3COOH��OH�� ��H2��0 ( K2��5.71��10��10 )
�����£���������������ʵ���Ũ�ȵĴ���ʹ�������Һ��ϣ�����������ȷ����( )
A�������ҺpH��7
B���Ի����Һ�����ȣ�K1����K2��С
C�����ڻ����Һ��pH�����ԣ����ʱ��Һ��c(Na��)��c(CH3COO��)
D���¶Ȳ��䣬���ڻ����Һ�м�������NaOH���壬c(CH3COO��)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�Դ���ƽ��״̬�ķ�Ӧ2A(g)��B(g) 2C(g) ��H��0������������ȷ���� ��
2C(g) ��H��0������������ȷ���� ��  ��
��
A�������¶ȣ�v����С��v������ B������AŨ�ȵ�˲�䣬v������v�治��
C������ѹǿ��v������v���С D��������������v������v���С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������H�������( )
A��Ba(OH)2��NH4Cl������ B���Ȼ�立ֽ�ð���
C��̼��Ʒֽ�ö�����̼ D��ʵ�����Ʊ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������������ǽ������˸���12���¿���ѧ�Ծ��������棩 ���ͣ������
��ˮ�DZ������Դ���⣬Ŀǰ�ȼҵ����ˮ��þ����ˮ����Ϊ�����ṩ�˴�����ҵԭ�ϡ���ͼ�Ǻ�ˮ�ۺ����õIJ�������ͼ����ͼ�ش����⣺

��1���ɺ�ˮɹ�ƵĴ����к� ��Ca2+��Mg2+��SO42�������ӣ�Ϊ��ȥ��Щ���ӣ������Լ������Ⱥ�˳��Ϊ(д��ѧʽ) ___________________��
��Ca2+��Mg2+��SO42�������ӣ�Ϊ��ȥ��Щ���ӣ������Լ������Ⱥ�˳��Ϊ(д��ѧʽ) ___________________��
��2���ٵ�ⱥ��ʳ��ˮ�Ļ�ѧ��Ӧ����ʽΪ______________��
����ȡMgCl2�Ĺ������漰��Ӧ��MgC l2��6H2O
l2��6H2O MgCl2+6H2O���÷�ӦҪ��HCl�����н��У�ԭ����_______________��
MgCl2+6H2O���÷�ӦҪ��HCl�����н��У�ԭ����_______________��
��3����±��ͨ��Cl2�û���Br2����������SO2���գ�д����SO2���շ��������ӷ���ʽ_________���ɴ��ж�Cl2��Br2��SO2����������ǿ������˳��Ϊ__________________��
��4��Ҳ�й������ڴ���Br2����̼������Һ���գ��γ��廯�ƺ������ƣ�ͬʱ��CO2�ų����÷�Ӧ�����ӷ���ʽ��_____________���������H2SO4�����õ�Br2��֮�����CCl4����Br2����ȡ��������_____________�����õ�����Br2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��У�����������ѧ�ڵڶ���������ѧ�Ծ��������棩 ���ͣ�ѡ����
�谢���ӵ�������ֵΪNA��������˵����ȷ����
A����״���£�22.4 L��ϩ���еĹ��õ��Ӷ���Ϊ5NA
B��1 mol NaHSO4�е���������Ϊ2NA
C��ͨ��״���£�1 mol NO��0.5 molO2 ���ܱ������л�ϣ�����NO2������ΪNA
D����ȡƯ��ʱ����״����22.4 LCl2 �μӷ�Ӧ��ת�Ƶ�����ΪNA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com