
��10�֣�������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý�������ˮ���е�NO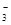 ��ԭΪN2���Ӷ�������Ⱦ���䷴Ӧ�����ӷ���ʽ�ǣ�
��ԭΪN2���Ӷ�������Ⱦ���䷴Ӧ�����ӷ���ʽ�ǣ�
6NO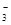 +10Al+18H2O��3N2��+10Al��OH��3+6OH-
+10Al+18H2O��3N2��+10Al��OH��3+6OH-
��1��������Ӧ�У����ɱ�״����33��6L����ʱ��ת���ϵ���Ϊ
mol������Ҫ��ȥ1m3����Ԫ��0��3mol�ķ�ˮ�е�N0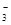 ���赪Ԫ�ض���NO
���赪Ԫ�ض���NO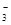 ����ʽ���ڣ���ͬ������������Ҫ���Ľ�����
g��
����ʽ���ڣ���ͬ������������Ҫ���Ľ�����
g��
��2��������Ϊ����þ�����ܸ�������������Ⱦ���䷴Ӧԭ���ͽ�������ͬ����
��д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ
����֪����þ���ԴӺ�ˮ����ȡ��MgCI2ͨ������Ƶõģ���������Ȼ�þ�Ļ�ѧ����ʽΪ
����Ҫ��ȥ1m3����Ԫ��0.3mol�ķ�ˮ�е�NO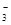 ����������Ҫ��0��5%������������MgCl2�ĺ�ˮ kg��
����������Ҫ��0��5%������������MgCl2�ĺ�ˮ kg��
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý�������ˮ���е�NO3����ԭΪN2���Ӷ�������Ⱦ���䷴Ӧ�����ӷ���ʽ�ǣ�
6NO3��+ 10Al + 18H2O �� 3N2��+ 10Al(OH)3+ 6OH��
��1������Ҫ��ȥ1m3����Ԫ��0.3mol�ķ�ˮ�е�NO3�����赪Ԫ�ض���NO3������ʽ���ڣ���ͬ������������Ҫ���Ľ�����__________g��
��2��������Ϊ����þ�����ܸ�������������Ⱦ���䷴Ӧԭ���ͽ�������ͬ��
�� д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ��___________________________________
�� ��֪����þ�ǴӺ�ˮ����ȡ��MgCl2��ͨ������Ƶõġ���Ҫ��ȥ1m3����Ԫ��0.3mol�ķ�ˮ�е�NO3������������Ҫ��0.5��������������MgCl2�ĺ�ˮ_________kg��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com