
����Ŀ��������������������о���ʮ����Ҫ�����ã���������ӵ����̫���ܵ��ռ�кܴ���ء�̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�á�������е����裬����ͭ���ࡢ�ء����Ȼ����
��1����̬��ͭ�����е���ռ�ݵ�ԭ�ӹ����ĿΪ____________��
��2������̬��ԭ�Ӽ۲�����Ų�ʽд��4s24px24py2������Υ����____________��
��3����ͼ��ʾ̼�������Ԫ�ص��ļ������ܱ仯���ƣ����б�ʾ��������_______(����)��

��4��Ԫ��X���ͬ������ԭ�Ӱ뾶��С��X�γɵ�����⻯��Q�ĵ���ʽΪ_____���÷���������ԭ�ӵ��ӻ�����Ϊ_____��д��һ����Q��Ϊ�ȵ����������______��
��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԡ���Ȼ���к���Ԫ�ص�������һ����Ȼ��أ��仯ѧʽд��Na2B4O7��10H2O��ʵ�������Ľṹ��Ԫ��������H3BO3������[B(OH)4]-���϶��ɵ�˫��Ԫ����Ӧ��д��Na2[B4O5(OH)4]8H2O����ṹ��ͼ��ʾ�����������ӿ��γ���״�ṹ����þ����в����ڵ���������__________(��ѡ����ĸ)��

A ���Ӽ� B ���ۼ� C ������ D ���»��� E ���
��6��GaAs���۵�Ϊ1238�棬�ܶ�Ϊ��g��cm3���侧���ṹ��ͼ��ʾ����֪GaAs��GaN������ͬ�ľ����ṹ������߾�������;�Ϊ____��GaAs���۵�____������������������������GaN��Ga��As��Ħ�������ֱ�ΪMGa gmol1��MAs gmol1��ԭ�Ӱ뾶�ֱ�ΪrGa pm��rAs pm�������ӵ�����ֵΪNA����GaAs������ԭ�ӵ����ռ��������İٷ���Ϊ_______��

���𰸡�14 ���ع��� b  sp3 NH4+ C ԭ�Ӿ��� ���� [4��10-30��NA��(r3Ga+r3As)]/3(MGa+M As)
sp3 NH4+ C ԭ�Ӿ��� ���� [4��10-30��NA��(r3Ga+r3As)]/3(MGa+M As)
��������
��1�����ݻ�̬��ͭ���Ӻ�������Ų�ʽ�����������
��2�����ݺ��ع���������ͬ�����Ĺ�����Ų�ʱ���������ܷ�ռ��ͬ�Ĺ����������������ͬ��
��3����ԭ�ӵ�һ���ڶ��������������Ƿֱ�ʧ3p����ϵ�1���������յ����������ĵ�����ʧ3s�����1���������յ�������
��4��Ԫ��X���ͬ������ԭ�Ӱ뾶��С��X��CԪ�أ�
��5������Na2[B4O5(OH)4]8H2O�Ľṹͼ����������������
��6��ԭ�Ӿ�����ص����۵�ߣ�ԭ�Ӿ�����ԭ�Ӱ뾶ԽС���۵�Խ�ߣ�������ԭ�ӵ����ռ��������İٷ���=������ԭ�ӵ�������¾��������100%��
��1����̬��ͭ���Ӻ�������Ų�ʽ��1s22s22p63s23p63d10��ռ�ݵ�ԭ�ӹ������14��
��2�����ݺ��ع���������ͬ�����Ĺ�����Ų�ʱ���������ܷ�ռ��ͬ�Ĺ����������������ͬ������̬��ԭ�Ӽ۲�����Ų�ʽд��4s24px24py2������Υ���˺���ԭ��
��3����ԭ��3p�������3�����ӣ���һ���ڶ��������������Ƿֱ�ʧ3p����ϵ�1���������յ����������ĵ�����ʧ3s�����1���������յ�������3s���Ϊȫ�����ȶ�״̬�����Ե��ĵ�������������������ϴ�̼�����һ���ڶ������ֱܷ�ʧ�����p���2���������յ�����������������ʧ�����s2��1���������յ������� s���Ϊȫ�����ȶ�״̬�����Ե�����������ڶ����������ϴ���������b��ʾ�ף�
��4��CԪ���γɵ�����⻯����CH4��CH4�ǹ��ۻ��������ʽΪ![]() ���÷���������ԭ��C���ӻ��������
���÷���������ԭ��C���ӻ��������![]() ���ӻ�����Ϊsp3��ԭ������ͬ���۵�����Ҳ��ͬ����Ϊ�ȵ����壬CH4��ԭ������5���۵�������8����CH4��Ϊ�ȵ������������NH4+��
���ӻ�����Ϊsp3��ԭ������ͬ���۵�����Ҳ��ͬ����Ϊ�ȵ����壬CH4��ԭ������5���۵�������8����CH4��Ϊ�ȵ������������NH4+��
��5�����ݽṹͼ��֪�����ý������������֮��������Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ�������֮����ڷ��»�����ˮ����֮�������������Բ����ڵ��������ǽ�������ѡC��
��6��GaAs���۵�Ϊ1238�棬�۵�ߣ���������ԭ�Ӿ��壻Asԭ�ӵİ뾶����Nԭ�ӣ�ԭ�Ӿ�����ԭ�Ӱ뾶ԽС���۵�Խ�ߣ�����GaAs���۵����GaN�����ݾ�̯ԭ��GaAs������Gaԭ�Ӹ�����4��Asԭ������![]() ��1��������ԭ�ӵ��������
��1��������ԭ�ӵ��������![]() �����ݾ����ܶȵļ��㹫ʽ��
�����ݾ����ܶȵļ��㹫ʽ��![]() ,
,![]()
![]() ��GaAs������ԭ�ӵ����ռ��������İٷ���Ϊ
��GaAs������ԭ�ӵ����ռ��������İٷ���Ϊ =[4��10-30��NA��(r3Ga+r3As)]/3(MGa+MAs)��
=[4��10-30��NA��(r3Ga+r3As)]/3(MGa+MAs)��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ2SO2��O2![]() 2SO3����������ȷ����(����)
2SO3����������ȷ����(����)
A.�÷�Ӧ�ǿ��淴Ӧ
B.��Ӧ����ʽ����![]() ����ʾ����ͬ�����£���Ӧ����ͬʱ�������������
����ʾ����ͬ�����£���Ӧ����ͬʱ�������������
C.1 mol O2��2 mol SO2��Ϸ�Ӧ��������2 mol SO3
D.�ڸ÷�Ӧ��SO2����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������þ��(Mg2B2O5��H2O����Fe2O3����)��ȡ����(H3BO3)������������¡�
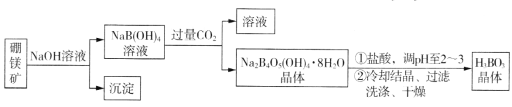
ͬ���������⣺
(1)��������Ҫ�ɷ�Ϊ____________________(�ѧʽ)��
(2)д������Na2B4O5(OH)4��8H2O�Ļ�ѧ����ʽ_________________________________��
(3)����H3BO3����ϴ�Ӹɾ��IJ�����______________________________��
(4)��֪��
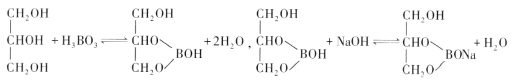
ʵ�������ô�ԭ���ⶨ������Ʒ�����������������ȷ��ȡ0.3000g��Ʒ����ƿ�У�����������ͼ���ʹ�����ܽⲢ��ȴ������1��2�η�̪��Һ��Ȼ����0.2000mol��L-1NaOH����Һ�ζ����յ㣬����NaOH��Һ22.00mL��
�ٵζ��յ������Ϊ________________________��
�ڸ�������Ʒ�Ĵ���Ϊ_________________��(����1λС��)��
(5)���NaB(OH)4��Һ�Ʊ�H3BO3�Ĺ���ԭ������ͼ��
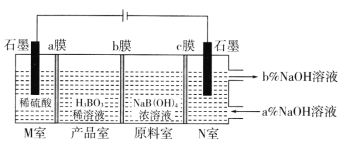
��bĤΪ________����Ĥ(������������������������)��������ÿ����1molH3BO3�������ҹ�����__________L����(��״��)��
��N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��_________b��(����>������<��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ��������˵������ȷ����( )
A���к��Ȳⶨʵ���У�ӦѸ�ٽ�NaOH��Һ�������ڲⶨװ���л��
B��Ϊ�ⶨ������ˮ��pH���ò�����պȡҺ�����pH��ֽ�ϣ������ɫ�����ռ���
C��̽��������H2O2�ֽ����ʵ�Ӱ�죺����ͬ�����£���һ֧�Թ��м���2mL 5%H2O2��1mL H2O������һ֧�Թ��м���2mL 5%H2O2��1mL FeCl3��Һ���۲첢�Ƚ�ʵ������
D����֪![]() ��Ϊ�����ø÷�Ӧ̽����Ӧ�������¶ȵĹ�ϵ�������Լ���1 mol��L-1KI��Һ��0.1 mol��L-1ϡ�����⣬����Ҫ�õ�������Һ
��Ϊ�����ø÷�Ӧ̽����Ӧ�������¶ȵĹ�ϵ�������Լ���1 mol��L-1KI��Һ��0.1 mol��L-1ϡ�����⣬����Ҫ�õ�������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʾ��ͼ���ʾ����
��Ӧ | ������ | �� | �� |
| |
A | �������ļء��Ʒֱ���ˮ��Ӧ | H2������ | �� | �� | |
B | ��ͬ�����İ�����ͬһ������������2NH3 | NH3��ת���� | 500 | 400 | |
C | �����Ϊ1:3��N2��H2������ɱ�ĺ�ѹ�����з���2NH3 | NH3��Ũ�� | ���ԸߵĴ��� | ����һ��Ĵ��� | |
D | 2molSO2��1molO2��ͬ���·���2SO2��O2 | SO3���ʵ��� | 2������ѹ | 10������ѹ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ױ����л��ϳɵ���Ҫԭ�ϣ��ȿ������ϳɿ����в�������ҩ����м���E��Ҳ�������ϳ���-���ڵ��ڼ���ҩ���м���K���ϳ�·�����£�

��֪����.R1NH2+Br-R2![]() R1-NH-R2+HBr
R1-NH-R2+HBr
��.![]()
��1��A�Ľṹ��ʽΪ______________��
��2��C�к�������������Ϊ_____________��
��3��C��D�Ļ�ѧ����ʽΪ_____________��
��4��F��G�ķ�Ӧ����Ϊ________________��
��5��H��I�Ļ�ѧ����ʽΪ________________��
��6��J�Ľṹ��ʽΪ_____________��
��7��������Ŀ������Ϣ����![]() ��
�� Ϊԭ�Ϻϳɻ�����L���������£�д���м����1���м����2�Ľṹ��ʽ��______��_________��
Ϊԭ�Ϻϳɻ�����L���������£�д���м����1���м����2�Ľṹ��ʽ��______��_________��
��
�ںϳ�L�Ĺ����л����ܵõ�һ�ָ߷��ӻ������ṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���谢���ӵ���������ֵΪNA������˵����ȷ����
A. 1 L 1 mol��L-1��NaHSO3��Һ�к��е�������Ϊ3NA
B. 5��6g��ϩ�ͻ�����Ļ�����к�C��H����ĿΪ0��8NA
C. ���³�ѹ�£�22��4L��37Cl2��������������Ϊ40NA
D. ������ͭ��Ӧ����0��1mol NOxʱ��ת�Ƶ�����Ϊ0��2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ1����2 mol SO2��1 mol O2�����������ɱ���ܱ������У���T1�¶��·������·�Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)����H��0������Ӧ���е�ʱ�� t0ʱ�ﵽƽ��״̬����û������������ʵ���Ϊ2.1 mol�� �Իش�
2SO3(g)����H��0������Ӧ���е�ʱ�� t0ʱ�ﵽƽ��״̬����û������������ʵ���Ϊ2.1 mol�� �Իش�

��1��t0ʱSO3�����ʵ���Ϊ��____________����ͼ2��������Ӧ������и������������ʵ����淴Ӧ���е�t0ʱ�ı仯����____________��
��2������Ӧ���е�t1ʱ��ʱ(t1��t0)����������Ӧ�������ȵ��¶�ΪT2 (T2��T1)����Ӧ��t3ʱ(t3��t1)���´ﵽƽ��״̬����ƽ������������������ʵ���________2.1 mol�����������������������
��3����ͼ1��ʾ������ʼʱ�������м���0.6 mol SO2��0.3 mol O2 �������¶Ȳ��䣬�ﵽƽ��״̬��SO3������������������ƽ�����__________�������������������������
��4��V2O5��������Ӧ�Ĵ�������ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ________��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼ��ʾװ����ȡ�����ı���֤���÷�Ӧ��ȡ����Ӧ����ش�

��1������A������Ϊ____________������ˮ�Ľ�ˮ��Ϊ_________��(����m������n��)��
��2����ȡ�屽�Ļ�ѧ����ʽΪ___________________��
��3����ƿ������NaOH��Һ��������_________��
��4����ʵ�鰲ȫ�ĽǶȷ�������ʵ��װ�ô���һ�����Ե�ȱ����ָ��_________��
��5����Ӧ������������ƿ�еμ�����������Һ�������Ȼ����__________(����������)������屽(�Ժ���������)��
��6�����ʵ��֤����ȡ�屽�ķ�Ӧ��ȡ����Ӧ___________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com