
С��ͬѧ������ͼ��ʾװ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
�밴Ҫ����գ�
 |

��1�����ö������̺�Ũ������ȡ�����������������Ҫ�õ�����������˳��Ϊ��
A�� ��
��2������Bװ�ÿ���ȡ�������� ����2�֣���
��3��B��D��Eװ����������B��ʢװ����Ũ�����ͭƬ�������п����ϰ��ϣ������Ƶ�NO2�������й�ʵ�顣
B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
����Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ�� ���ٴ�ֹˮ�� ��ʹ�ձ��е�ˮ�����Թܶ��IJ����� ��
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
С��ͬѧ������ͼ��ʾװ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У����밴Ҫ����գ�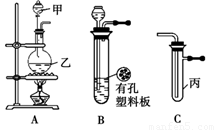
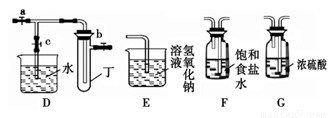
��1�����ö������̺�Ũ������ȡ�����������������Ҫ�õ�����������˳��Ϊ��A�� ��
��2������Bװ�ÿ���ȡ�������� ����2�֣���
��3��B��D��Eװ����������B��ʢװ����Ũ�����ͭƬ�������п����ϰ��ϣ������Ƶ�NO2�������й�ʵ�顣B�з�����Ӧ�Ļ�ѧ����ʽΪ ������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ�� ���ٴ�ֹˮ�� ��ʹ�ձ��е�ˮ�����Թܶ��IJ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com