
��״���£�1���ˮ�����ܽ�500�����HCl���塣����1 Lˮ��ͨ���״���µ�448LHCl���壬����������ȫ�ܽ⡣
��1����������Һ�ܶ�Ϊ1.2 g/cm3������Һ�к�HCl���ʵ���Ũ��Ϊ ��
��2���Ӹ���Һ��ȡ��10mLŨ�����ܽ���ˮ���Ƴ�500mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ��
��3������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ���������_______________��ѡ�����в�������ţ���
| A������ƿ����ˮϴ��δ�Ӹ��� |
| B����Ͳ������ˮϴ��δ���� |
| C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶��� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijѧ��������6��0 mol·L��1��H2SO4 1 000 mL��ʵ���������ֲ�ͬŨ�ȵ������480 mL 0��5 mol·L��1�������150 mL 25%������(�ѣ�1��18 g·mL��1)����������18 mol·L��1�����ᡣ�����ֹ�������ƿ��250 mL��500 mL��1 000 mL����ʦҪ��Ѣ٢���������ȫ�����꣬����IJ����ɢ������䡣
��ش��������⣺
��1��ʵ������25%����������ʵ���Ũ��Ϊ________mol·L��1(����1λС��)��
��2�����Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ________mL��
��3������ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ����� 18 mol·L��1��Ũ���� mL���ز����������������Һ�С�
���ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��______________________________________��
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2 cm����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
��4�����ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(�ƫ����ƫС������Ӱ�족)��
��5�����в���Cǰ����ע��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С����CaCl2��H2Ϊԭ�ϣ���ͼ�Ʊ� +1��Ca�Ļ����������ֲ�����ֻ�����ֻ�������ң���Ԫ����ɷ���������������иơ���Ԫ�ص����������ֱ�Ϊ52.36%��46.33%���������ҵ�ˮ��Һ�����ԡ���ش��������⣺
��1�����о�С���Ƿ�ɹ��Ƶ� +1��Ca�Ļ���� ����ǡ������Ļ�ѧʽ�� ��
��2������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�� ����Ӧ������Һ���ᾧ�ɵõ�һ�־��壬�仯ѧʽΪCaCl2�� xCa(OH)2�� 12H2O��Ϊȷ��x��ֵ�������ʵ�鷽�� ��
��3���ڼ��������£��ҵ�ˮ��Һ��Ũ����MnO2��Ӧ�����ӷ���ʽ�� ���ҵ�ˮ��Һ��Fe��Ӧ���õ���Һ���ȶ����������Һ�Ĵ�ʩ�� ��
��4����д��һ������Ϊ���ܵõ�CaCl�Ļ�ѧ����ʽ����CaCl2Ϊԭ�ϣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ���������
��1������һ����ɫ���ʻ�Ӫ��Һ������������ơ�̼��ء�����ء��Ȼ����е�һ�ֻ���������ɣ�Ϊ̽����ɷ֣�ijͬѧ��Ʋ����������ͼ��ʾ��ʵ�顣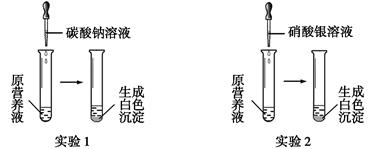
��������ʵ�飬������ա�
����ʵ��1��ȷ��ԭӪ��Һ��һ��û�е������� (�ѧʽ)��д�����ɰ�ɫ���������ӷ���ʽ�� ��
�������ԭӪ��Һ��K+��Cl-����Ŀ֮��Ϊ2��1����ԭӪ��Һ���� ���������Ƴɵġ�
��ijͬѧ���Ȼ��ơ�����ء��Ȼ�����ɵ�Ӫ��Һ��K+��Cl-��NO3-����Ŀ֮��Ϊ2��5��1��
����������غ��Ȼ��Ƶ����ʵ���֮���� ��
��2���±���500mLij���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⡣
| �ɷ� | ������g�� | Ħ��������g ��mol-1�� |
| ���� | 68.4 | 342 |
| ����� | 0.50 | 174 |
| ��˾ƥ�� | 0.35 | 180 |
| ������� | 0.50 | 158 |
| ������ | 0.04 | 170 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����ξ���KxFey(C2O4)z?wH2O����Ϊ+3�ۣ������Ϊ-2�ۣ���֪x+y+z=7��ȡ�þ�����������ʵ�飺
�� ȡ4��910g�����ڲ�ͬ�¶��¼��������أ����ù���Ļ�ѧʽ���������±���
| | 120�� | 300�� | 480�� |
| ��ѧʽ | KxFey(C2O4)z | KxFeyO(C2O4)z-1 | KxFeyO2(C2O4)z-2 |
| ���� | 4��370g | 3��650g | 2��930 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��֪��������[NaxFey(SO4)m(OH)n]���г��������������ʿ졢�����˵��ص㡣ij�о�С���Ƚ�ij��ˮ��Fe2������ΪFe3�����ټ���Na2SO4ʹ�����ɻ�����������ȥ����С��Ϊ�ⶨ������������ɣ�����������ʵ�飺
�ٳ�ȡ4.850 g��Ʒ����������ȫ�ܽ�����100.00 mL��ҺA��
����ȡ25.00 mL��ҺA������������KI����0.2500 mol��L��1Na2S2O3��Һ���еζ�����ӦΪI2��2Na2S2O3��2NaI��Na2S4O6��������30.00 mLNa2S2O3��Һ���յ㡣
����ȡ25.00 mL��ҺA��������BaCl2��Һ��ַ�Ӧ���ˡ�ϴ�ӡ������ó���1.165 g��
��1����С�鲻��������Fe(OH)3�����ķ�����ȥ��Ԫ�أ�����Ϊ���ɵ�Fe(OH)3 ��
��2����Na2S2O3��Һ���еζ�ʱ��ʹ�õ�ָʾ��Ϊ ���ζ����յ����ɫ�仯Ϊ ��
��3��ͨ������ȷ�����������Ļ�ѧʽ��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��0.1 mol��þ�����Ļ��������50 mL 4 mol��L-1 H2SO4��Һ�У�Ȼ���ٵμ�2 mol��L-1��NaOH��Һ����ش��������⣺
��1�����ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�ı仯��ͼ��ʾ����V1��80 mLʱ�����������ĩ��þ�����ʵ�����V2�������
��2�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2����Al3���պó�����ȫ���������NaOH��Һ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��5.0g̼��Ʒ���һ����ϡ������ǡ����ȫ��Ӧ���õ�40mL�ܶ�Ϊ1.32g/mL����Һ���Լ��㣺
��1�����ɱ�״���µĶ�����̼�������������������CO2��ˮ�е��ܽ⣩
��2��������Һ���Ȼ��Ƶ����ʵ���Ũ�ȡ�
��3������ϡ���������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com