
ŅĄ¾ŻŠšŹö£¬Š“³öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½”£
£Ø1£©ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌŚC2H2(ĘųĢ¬)ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®µÄ·“Ó¦ÖŠ£¬ĆæÓŠ4NAøöµē×Ó×ŖŅĘŹ±£¬·Å³ö450 kJµÄČČĮ攣ĘäČČ»Æѧ·½³ĢŹ½ĪŖ______________________”£
£Ø2£©ŅŃÖŖ²šæŖ1 mol H”ŖH¼ü”¢1 mol N”ŖH¼ü”¢1 mol N”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436 kJ”¢395 kJ”¢940 kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
£Ø3£©īŃ£ØTi£©±»³ĘĪŖ¼ĢĢś”¢ĀĮÖ®ŗóµÄµŚČż½šŹō£¬ŅŃÖŖÓɽšŗģŹÆ£ØTiO2£©ÖĘČ”µ„ÖŹTi£¬Éę¼°µÄ²½ÖčĪŖ£ŗ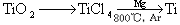
ŅŃÖŖ£ŗ¢ŁC(s)+O2(g) 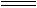 CO2(g); ¦¤H=£395.5 kJ”¤mol-1
CO2(g); ¦¤H=£395.5 kJ”¤mol-1
¢Ś2CO(g)+O2(g) 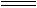 2CO2(g); ¦¤H=£560 kJ”¤mol-1
2CO2(g); ¦¤H=£560 kJ”¤mol-1
¢ŪTiO2(s)+2Cl2(g)+2C(s) 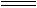 TiCl4(s)+2CO(g)µÄ¦¤H=ØD80kJ/mol
TiCl4(s)+2CO(g)µÄ¦¤H=ØD80kJ/mol
ŌņTiO2(s)ÓėCl2(g)·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø13·Ö£©
(1)C2H2(g)+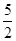 O2(g)£½2CO2(g)+ H2O(l) ¦¤H£½£1125 kJ/mol £Ø4·Ö£©
O2(g)£½2CO2(g)+ H2O(l) ¦¤H£½£1125 kJ/mol £Ø4·Ö£©
(2)N2(g)+ 3H2(g)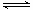 2NH3(g) ¦¤H£½£122 kJ/mol £Ø4·Ö£©
2NH3(g) ¦¤H£½£122 kJ/mol £Ø4·Ö£©
(3)TiO2(s)+ 2Cl2(g)£½TiCl4(s)+ O2(g) ¦¤H£½£«151 kJ/mol £Ø5·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŅŅČ²·Ö×ÓÖŠĢ¼ŌŖĖŲµÄ»ÆŗĻ¼ŪŹĒ£1¼Ū£¬·“Ó¦ŗó±äĪŖ£«4¼Ū£¬Ź§Č„5øöµē×Ó£¬¼“1molŅŅČ²Ź§Č„10molµē×Ó£¬ŌņĆæÓŠ4NAøöµē×Ó×ŖŅĘŹ±£¬ĻūŗÄŅŅČ²µÄĪļÖŹµÄĮæŹĒ0£®4mol£¬ĖłŅŌĆæĻūŗÄ1molŅŅČ²·Å³öµÄČČĮæŹĒ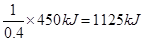 £¬Ņņ“ĖøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒC2H2(g)+
£¬Ņņ“ĖøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒC2H2(g)+ 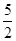 O2(g)£½2CO2(g)+ H2O(l) ¦¤H£½£1125 kJ/mol”£
O2(g)£½2CO2(g)+ H2O(l) ¦¤H£½£1125 kJ/mol”£
£Ø2£©·“Ó¦ČČ¾ĶŹĒ¶Ļ¼üĪüŹÕµÄÄÜĮ棬ŗĶŠĪ³É»Æѧ¼üĖł·Å³öµÄÄÜĮæµÄ²īÖµ£¬Ōņøł¾Ż¼üÄÜæÉÖŖ£¬ĆæÉś³É2mol°±ĘųµÄ·“Ó¦ČČ”÷H£½436kJ/mol”Į3£«940kJ/mol£2”Į3”Į395kJ/mol£½£122 kJ/mol£¬¼“·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒN2(g)+ 3H2(g)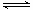 2NH3(g) ¦¤H£½£122 kJ/mol”£
2NH3(g) ¦¤H£½£122 kJ/mol”£
£Ø3£©øł¾ŻøĒĖ¹¶ØĀÉæÉÖŖ£¬¢Ū£«¢Ś£¢Ł”Į2£¬¼“µĆµ½·“Ó¦TiO2(s)+ 2Cl2(g)£½TiCl4(s)+ O2(g) £¬ĖłŅŌøĆ·“Ó¦µÄ·“Ó¦ČȦ¤H£½ØD80kJ/mol£560 kJ/mol£«395£®5 kJ/mol”Į2£½£«151 kJ/mol”£
æ¼µć£ŗæ¼²éČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ŅŌ¼°·“Ó¦ČȵÄÓŠ¹Ų¼ĘĖć”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŅŃÖŖ£ŗ¢Ł1 mol H2·Ö×ÓÖŠ»Æѧ¼ü¶ĻĮŃŹ±ŠčŅŖĪüŹÕ436 kJµÄÄÜĮ棬¢Ś1 mol I2ÕōĘųÖŠ»Æѧ¼ü¶ĻĮŃŹ±ŠčŅŖĪüŹÕ151 kJµÄÄÜĮ棬¢ŪÓÉHŌ×ÓŗĶIŌ×ÓŠĪ³É1 mol HIĘųĢ¬·Ö×ÓŹ±ŹĶ·Å299 kJµÄÄÜĮ攣ĻĀĮŠČČ»Æѧ·½³ĢŹ½ÕżČ·µÄŹĒ
| A£®2HI(g) =H2(g)£«I2(g)?H£½+11 kJ£Æmol |
| B£®H2(g)£«I2(g) =2HI(g)?H£½£22 kJ£Æmol |
| C£®H2(g)£«I2(g) =2HI(g)?H£½+288 kJ£Æmol |
| D£®H2(g)£« I2(g) =2HI(g)?H£½£144 kJ£Æmol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŅŃÖŖ£ŗ2H2(g)£«O2(g)=2H2O(g) ¦¤H£½£483.6 kJ”¤mol£1
H2(g)£«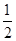 O2(g)=H2O(l) ¦¤H£½£285.8 kJ”¤mol£1
O2(g)=H2O(l) ¦¤H£½£285.8 kJ”¤mol£1
ÓÉ“ĖæÉÖŖ£¬ŌŚµČĪĀĻĀÕō·¢36 gŅŗĢ¬Ė®ŠčĪüŹÕµÄČČĮæ£Ø £©
| A£®483.6 kJ | B£®88 kJ | C£®285.8 kJ | D£®44 kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
·ÖĪöÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĻĀĮŠŃ”ĻīÕżČ·µÄŹĒ
| A£®S£Øs£¬µ„Š±£©+O2£Øg£©=SO2£Øg£©”÷H ="+297.16" kJ?mol-1 |
| B£®S£Øs£¬µ„Š±£©+O2£Øg£©=SO2£Øg£©”÷H ="-296.83" kJ?mol-1 |
| C£®S£Øs£¬Õż½»£©+O2£Øg£©=SO2£Øg£©”÷H ="-296.83" kJ?mol-1 |
| D£®µ„Š±Įņ±ČÕż½»ĮņøüĪČ¶Ø |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ·“Ó¦ÖŠ¾É»Æѧ¼ü¶ĻĮŃĪüŹÕµÄ×ÜÄÜĮæ“óÓŚŠĀ»Æѧ¼üŠĪ³É·Å³ö×ÜÄÜĮæµÄŹĒ
| A£®µē½āĖ®ÖĘČ”H2ŗĶO2 | B£®¼×ĶéČ¼ÉÕ |
| C£®ĀĮ·ŪÓėŃõ»ÆĢś·ŪÄ©·“Ó¦ | D£®ÓĶÖ¬ŌŚČĖĢåÄŚĶźČ«Ńõ»ÆÉś³ÉCO2ŗĶH2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŠĪ³É½ŚŌ¼ÄÜŌ“ŗĶ±£»¤ÉśĢ¬»·¾³µÄ²śŅµ½į¹¹ŹĒČĖĄąÓė×ŌČ»ŗĶŠ³·¢Õ¹µÄÖŲŅŖ±£Ö¤£¬ÄćČĻĪŖĻĀĮŠŠŠĪŖÖŠÓŠć£ÓŚÕāŅ»±£Ö¤µÄŹĒ£Ø £©
| A£®ŃŠ¾æ²ÉĆŗ”¢²ÉÓĶŠĀ¼¼Źõ£¬Ģįøß²śĮæŅŌĀś×ć¹¤ŅµÉś²śµÄæģĖŁ·¢Õ¹ |
| B£®æŖ·¢Ģ«ŃōÄÜ”¢Ė®ÄÜ”¢·ēÄܵȊĀÄÜŌ“£¬¼õÉŁŹ¹ÓĆĆŗ”¢ŹÆÓĶµČ»ÆŹÆČ¼ĮĻ |
| C£®ŌŚÅ©“åĶĘ¹ćŹ¹ÓĆÕÓĘų |
| D£®¼õɣ׏Ō“ĻūŗÄ”¢Ōö¼Ó׏Ō“µÄÖŲø“Ź¹ÓĆŗĶ׏Ō“µÄŃ»·ŌŁÉś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ·“Ó¦ÖŠµÄÄÜĮæ±ä»Æ¹ŲĻµ·ūŗĻČēĶ¼ĖłŹ¾µÄŹĒ
| A£®ŃĪĖįÓėÉÕ¼ī·“Ó¦ |
| B£®ĢģČ»ĘųČ¼ÉÕ |
| C£®ČżŃõ»ÆĮņÓėĖ®·“Ó¦ |
| D£®ģŃÉÕŹÆ»ŅŹÆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŅŃÖŖ2H2(g)+O2(g) £½2H2O(l£©”÷H£½”Ŗ571.6kJ”¤mol”Ŗ1£¬2H2(g)+O2(g)£½2H2O(g£©”÷H£½”Ŗ483.6kJ”¤mol”Ŗ1”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®1molH2O(l)±ä³É1mo1H2O(g)£¬ĪüŹÕ88 kJÄÜĮæ |
| B£®1 molH2O(g)·Ö½ā³ÉH2(g)ŗĶO2 (g)£¬ĪüŹÕ241.8kJÄÜĮæ |
| C£®2 molH2(g)ŗĶ1molO2(g)µÄ×ÜÄÜĮæŠ”ÓŚ2molH2O(l)µÄÄÜĮæ |
| D£®æÉŅŌŃ°ÕŅĢŲŹā“߻ƼĮŹ¹H2O·Ö½ā£¬Ķ¬Ź±·Å³öÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ
| A£®NH4Cl(s)£½NH3(g)+HCl(g)ŹŅĪĀĻĀ²»ÄÜ×Ō·¢½ųŠŠ£¬ĖµĆ÷øĆ·“Ó¦µÄ¦¤H£¼0 |
| B£®¶ĘŠæĢśÖĘĘ·¶Ę²ćĘĘĖšŗó£¬ĢśÖĘĘ·±ČŹÜĖšĒ°øüČŻŅ×ÉśŠā£¬¶ų¶ĘĪżĢśŌņĻą·“ |
C£®¶ŌÓŚN2(g)+3H2(g) 2NH3(g)£¬µ±ĘäĖūĢõ¼ž²»±äŹ±£¬Ń¹ĖõĘųĢåĢå»żŹ¹Ń¹ĒæŌö“ó£¬Õż·“Ó¦ŗĶÄę·“Ó¦ĖŁĀŹŅŌ¼°H2µÄĘ½ŗā×Ŗ»ÆĀŹ¾łŌö“ó 2NH3(g)£¬µ±ĘäĖūĢõ¼ž²»±äŹ±£¬Ń¹ĖõĘųĢåĢå»żŹ¹Ń¹ĒæŌö“ó£¬Õż·“Ó¦ŗĶÄę·“Ó¦ĖŁĀŹŅŌ¼°H2µÄĘ½ŗā×Ŗ»ÆĀŹ¾łŌö“ó |
| D£®100”ꏱĖ®µÄĄė×Ó»ż³£ŹżKwĪŖ5.5”Į10£13£¬ĖµĆ÷Ė®µÄµēĄėŹĒ·ÅČČ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com