
| ŹµŃé²½Öč | ŹµĻÖĻÖĻó |
| £Ø1£©Č”ÉŁĮæøĆČÜŅŗ£¬¼Ó¼øµĪ¼×»ł³Č | ČÜŅŗ±äŗģÉ« |
| £Ø2£©Č”ÉŁĮæøĆČÜŅŗ¼ÓČČÅØĖõ£¬¼ÓCuʬŗĶ ÅØH2SO4£¬¼ÓČČ | ÓŠĪŽÉ«ĘųĢå²śÉś£¬ĘųĢåÓöæÕĘųÖŠŅŌ±ä³Éŗģ×ŲÉ« |
| £Ø3£©Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓBaCl2ČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É |
| £Ø4£©Č”£Ø3£©ÖŠÉĻ²ćĒåŅŗ£¬¼ÓAgNO3ČÜŅŗ | ÓŠĪČ¶ØµÄ°×É«³ĮµķÉś³É£¬ĒŅ²»ČÜÓŚHNO3 |
| £Ø5£©Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓNaOHČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É£¬µ±NaOH¹żĮæŹ±³Įµķ²æ·ÖČܽā |
·ÖĪö ŅņŹĒĪŽÉ«ĶøĆ÷ČÜŅŗ£¬Ōņ²»ŗ¬ÓŠFe2+£¬ÓÉ£Ø1£©ÖŖČÜŅŗĻŌĖįŠŌ£¬Ōņ²»“ęŌŚHCO3-£»ÓÉ£Ø2£©ÖŖÓŠNOÉś³É£¬ŌČÜŅŗÖŠŗ¬NO3-£¬ŌņŅ»¶Ø²»ŗ¬Fe2+”¢I-£Ø¾ßÓŠ»¹ŌŠŌ£©£»ÓÉ£Ø3£©ÖŖÓŠSO42-“ęŌŚ£¬ŌņŌČÜŅŗ²»ŗ¬Ba2+£»ÓÉ£Ø4£©²»ÄÜČ·¶ØŹĒ·ńŗ¬Cl-£¬Ņņ£Ø3£©ŅżČėCl-£»ÓÉ¢ŻÖŖŗ¬Mg2+”¢Al3+£®
½ā“š ½ā£ŗŅņŹĒĪŽÉ«ĶøĆ÷ČÜŅŗ£¬Ōņ²»ŗ¬ÓŠFe2+£¬
øł¾Ż£Ø1£©Č”ÉŁĮæøĆČÜŅŗ£¬¼Ó¼øµĪ¼×»ł³ČŹŌŅŗ£¬ČÜŅŗĻŌŗģÉ«£¬ĖµĆ÷ČÜŅŗĻŌŹ¾ĖįŠŌ£¬ĖłŅŌHCO3-²»“ęŌŚ£»
øł¾Ż£Ø2£©Č”ÉŁĮæøĆČÜŅŗ¼ÓČČÅØĖõ£¬¼ÓCuʬŗĶÅØĮņĖį£¬¼ÓČČÓŠĪŽÉ«ĘųĢå²śÉś£¬ĘųĢå£ØNO£©ÓöæÕĘųæÉŅŌ±ä³Éŗģ×ŲÉ«£Ø¶žŃõ»ÆµŖ£©£¬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠNO3-£¬ŌņŅ»¶Ø²»ŗ¬ÓŠI-£»
øł¾Ż£Ø3£©Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬Ōņŗ¬ÓŠSO42-£»
øł¾Ż£Ø4£©Č”£Ø3£©ÖŠµÄÉĻ²ćĒåŅ¹£¬¼ÓAgNO3£¬ÓŠĪČ¶ØµÄ°×É«³ĮµķÉś³É£¬ĒŅ²»ČÜÓŚĻ”ĻõĖį£¬Ōņ£Ø3£©ÉĻĒåŅŗÖŠŗ¬ÓŠCl-£¬ÓÉÓŚøł¾Ż£Ø3£©ÖŠ¼ÓČėĮĖĀČ»Æ±µ£¬ŅżČėCl-£¬²»ÄÜČ·¶ØŌČÜŅŗÖŠŹĒ·ń“ęŌŚCl-£»
øł¾Ż£Ø5£©Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓČėNaOHČÜŅŗÓŠ°×É«³ĮµķÉś³É£¬µ±NaOH¹żĮæŹ±£¬³Įµķ²æ·ÖČܽā£¬ŌņŌČÜŅŗÖŠŗ¬ÓŠMg2+”¢Al3+£»
£Ø1£©×ŪÉĻæÉÖŖ£¬ČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓŹĒ£ŗNO3-”¢SO42- Mg2+”¢Al3+£¬ČÜŅŗÖŠæĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒ£ŗI-”¢Ba2+”¢Fe2+”¢HCO3-£¬²»ÄÜČ·¶ØµÄÓŠ£ŗK+”¢Cl-£¬
¹Ź“š°øĪŖ£ŗNO3-”¢SO42- Mg2+”¢Al3+£»I-”¢Ba2+”¢Fe2+”¢HCO3-£»K+”¢Cl-£»
£Ø2£©ČÜŅŗÖŠµÄæÉÄÜ“ęŌŚµÄŅõĄė×ÓŹĒCl-£¬¼ģŃé·½·ØŹĒĪŖ£ŗȔɣĮæŌČÜŅŗ¼Ó×ćĮæµÄĻõĖį±µČÜŅŗ£¬¹żĀĖŗó£¬ĻņĀĖŅŗÖŠ¼ÓĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬ŌŁ¼ÓĻ”ĻõĖį£¬°×É«³Įµķ²»Čܽā£¬ĖµĆ÷ÓŠCl-£¬·“Ö®Ōņƻӊ£¬
¹Ź“š°øĪŖ£ŗȔɣĮæŌČÜŅŗ¼Ó×ćĮæµÄĻõĖį±µČÜŅŗ£¬¹żĀĖŗó£¬ĻņĀĖŅŗÖŠ¼ÓĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬ŌŁ¼ÓĻ”ĻõĖį£¬°×É«³Įµķ²»Čܽā£¬ĖµĆ÷ÓŠCl-£¬·“Ö®Ōņƻӊ£®
µćĘĄ ±¾Ģā漲鳣¼ūĄė×ӵļģŃéŗĶ¼ų±š£¬ŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶ£¬×ŪŗĻŠŌĒ棬ӊĄūÓŚÅąŃųѧɜµÄĀß¼ĶĘĄķÄÜĮ¦ŗĶŹµŃéÉč¼ĘÄÜĮ¦£¬ÕĘĪÕŌŖĖŲ»ÆŗĻĪļŠŌÖŹŹĒ¹Ų¼ü£¬×¢ŅāÕĘĪÕ³£¼ūĄė×ӵļģŃ飬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
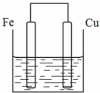 ½«ÖŹĮæĻąµČµÄĢśĘ¬ŗĶĶʬÓƵ¼ĻßĻąĮ¬½žČė500mLĮņĖįĶČÜŅŗÖŠ¹¹³ÉČēĶ¼µÄ×°ÖĆ£ŗ£ØŅŌĻĀ¾ł¼ŁÉč·“Ó¦¹ż³ĢÖŠČÜŅŗĢå»ż²»±ä£©£®
½«ÖŹĮæĻąµČµÄĢśĘ¬ŗĶĶʬÓƵ¼ĻßĻąĮ¬½žČė500mLĮņĖįĶČÜŅŗÖŠ¹¹³ÉČēĶ¼µÄ×°ÖĆ£ŗ£ØŅŌĻĀ¾ł¼ŁÉč·“Ó¦¹ż³ĢÖŠČÜŅŗĢå»ż²»±ä£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| X | Y | ||
| Z | W |
| A£® | ³£ĪĀĻĀ£¬ĖÄÖÖŌŖĖŲµ„ÖŹÖŠ£¬Č«²æŹĒ¹ĢĢå | |
| B£® | ZµÄŃōĄė×ÓÓėYµÄŅõĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬ | |
| C£® | XµÄĘųĢ¬Ēā»ÆĪļ±ČYµÄĘųĢ¬Ēā»ÆĪļĪČ¶Ø | |
| D£® | WŌŖĖŲŌ×Ó°ė¾¶±ČZŌŖĖŲŌ×Ó°ė¾¶“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4+K+NO3-Cl- | B£® | NO3-CO32-K+Na+ | ||
| C£® | K+Na+ Cl-SO42- | D£® | Mg2+Cu2+SO42-Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH2O | B£® | C2H4O | C£® | C2H4O2 | D£® | C3H8O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±½·Ö×ӵĽį¹¹ÖŠĮłøöĢ¼Ō×ÓµÄĮ¬½Ó·½Ź½ŹĒµ„Ė«½Ø½»Ģę×é³ÉµÄ»·×“ | |
| B£® | ±½ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬ĖłŅŌ±½ŹōÓŚĻ©Ģž | |
| C£® | ±½·Ö×ÓÖŠ6øöĢ¼Ģ¼»Æѧ¼üĶźČ«ĻąĶ¬ | |
| D£® | ±½æÉŅŌÓėäåĖ®”¢øßĆĢĖį¼ŲČÜŅŗ·“Ó¦¶ųŹ¹ĖüĆĒĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČ»ÆÄĘ | B£® | °±Ęų | C£® | NH4ClµÄµē×ÓŹ½  | D£® | Įņ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com