
����ѧ�����ʽṹ�����ʡ�
�����ס�����ͬ��Ԫ�أ�����Ԫ�ص��ʼ��仯������ũҩ�����ʵȷ�������Ҫ���á�
��ش��������⡣
��1����ԭ����Χ�����Ų�ʽΪ ��
��2���۷е㣺NH3 PH3��>��<��=��ԭ����
��3��N��P��As����Ԫ�ص縺����С�����˳��Ϊ ��NCl3����������ԭ�ӵ��ӻ���ʽΪ ��
��4������β����CO��NO���������ñ��N2��CO2���˷�Ӧ������Ԫ�ص�һ��������С�����˳��Ϊ ���������������к��м��Լ��ķǼ������� ����
���� ���ı�Ϊ
��
���ı�Ϊ
��
��5��K3[Fe��CN��6]�������������������� ����λ��Ϊ ��
A����� B�����Ӽ� C�����ۼ� D����λ��[ E�����»���
��6��CO��NH3�����ṩ�µ��Ӷ���Cu+�γ�����Cu+��NH3�γɵ������ɱ�ʾΪ
[Cu��NH3��n]+����������У�Cu+��4s�����4p���ͨ��sp�ӻ�����NH3�ṩ�ŵ��Ӷԣ���[Cu��NH3��n]+��n��ֵΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| I1/kJ?mol-1 | I2/kJ?mol-1 |
I3/kJ?mol-1 |
I4/kJ?mol-1 |
I5/kJ?mol-1 |
| 738 | 1451 | 7733 | 10540 | 13630 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ�
��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
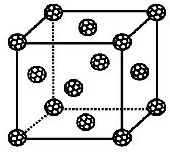 ����ѧ-���ʽṹ�����ʡ�
����ѧ-���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
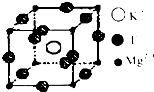 ����ѧ--���ʽṹ�����ʡ�
����ѧ--���ʽṹ�����ʡ�- 2 |
- 3 |
- 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com