
| A£® | ČÜŅŗÖŠĖÄÖÖĮ£×ÓÖ®¼äæÉÄÜĀś×ć£ŗc£ØNa+£©£¾c£ØOH-£©£¾c£ØH+£©£¾c£ØCH3COO-£© | |
| B£® | ČōČÜŅŗÖŠ²æ·ÖĮ£×Ó¼äĀś×ć£ŗc£ØCH3COO-£©=c£ØNa+£©£¬ŌņøĆČÜŅŗŅ»¶Ø³ŹÖŠŠŌ | |
| C£® | ČōČÜŅŗÖŠČÜÖŹ½öĪŖCH3COONa£¬ŌņĮ£×Ó¼äŅ»¶ØĀś×ć£ŗc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£© | |
| D£® | ČōČÜŅŗÖŠµÄČÜÖŹĪŖCH3COONaŗĶCH3COOH£¬ŌņČÜŅŗÖŠĮ£×Ó¼äæÉÄÜĀś×ć£ŗc£ØCH3COO-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£© |
·ÖĪö A£®øł¾ŻµēŗÉŹŲŗćæÉÖŖ£¬c£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£©ŹĒÓŠæÉÄܵģ»
B£®øł¾ŻµēŗÉŹŲŗćæÉÅŠ¶Ļc£ØNa+£©+c£ØH+£©=c£ØOH-£©+c£ØCH3COO-£©£»
C£®ČōČÜŅŗÖŠČÜÖŹ½öĪŖCH3COONa£¬ŌņĖ®½āĻŌ¼īŠŌ£»
D£®ČōČÜŅŗÖŠµÄČÜÖŹĪŖCH3COONaŗĶCH3COOHŹ±£¬ÓÉÓŚ²»ÄÜČ·¶ØŹĒĖ®½ā³Ģ¶Č“óÓŚµēĄė³Ģ¶Č»¹ŹĒµēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬ĖłŅŌČÜŅŗµÄĖį¼īŠŌĪŽ·ØČ·¶Ø£¬ČēCH3COOH½Ļ¶ą£¬ŌņČÜŅŗæÉÄܳŹĖįŠŌ£®
½ā“š ½ā£ŗA£®ČēČÜŅŗÖŠNaOH¹żĮ棬ĒŅ¼īŠŌ½ĻĒ棬ŌņæÉ“ęŌŚc£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¬¹ŹA“ķĪó£»
B£®ČÜŅŗ“ęŌŚµēŗÉŹŲŗćc£ØNa+£©+c£ØH+£©=c£ØOH-£©+c£ØCH3COO-£©£¬ČōĀś×ć£ŗc£ØCH3COO-£©=c£ØNa+£©£¬Ōņc£ØH+£©=c£ØOH-£©£¬øĆČÜŅŗŅ»¶Ø³ŹÖŠŠŌ£¬¹ŹBÕżČ·£»
C£®ČōČÜŅŗÖŠČÜÖŹ½öĪŖCH3COONa£¬ŌņĖ®½āĻŌ¼īŠŌ£¬Ó¦“ęŌŚc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£©£¬¹ŹCÕżČ·£»
D£®ČēCH3COOH½Ļ¶ą£¬ŌņČÜŅŗæÉÄܳŹĖįŠŌ£¬æÉÄÜĀś×ć£ŗc£ØCH3COO-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£©£¬¹ŹDÕżČ·£»
¹ŹŃ”A£®
µćĘĄ ±¾Ģāæ¼²éĄė×ÓÅØ¶ČµÄ“óŠ”±Č½ĻŅŌ¼°Čõµē½āÖŹµÄµēĄėµČĪŹĢā£¬ĢāÄæÄѶČÖŠµČ£¬×¢Ņāøł¾ŻČÜŅŗĄė×ÓÅØ¶ČµÄ¹ŲĻµ½įŗĻČõµē½āÖŹµÄµēĄėŗĶŃĪĄąµÄĖ®½āµČÖŖŹ¶·ÖĪö½ā“š£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒąĶ | B£® | øÖ | C£® | Ó²ĀĮ | D£® | ²£Į§ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
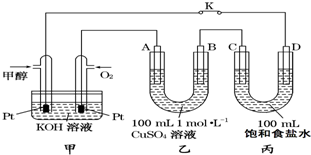 ČēĶ¼ŹĒ¼×“¼£ØCH3OH£©Č¼ĮĻµē³Ų¹¤×÷µÄŹ¾ŅāĶ¼£¬ĘäÖŠA”¢B”¢D¾łĪŖŹÆÄ«µē¼«£¬CĪŖĶµē¼«£®¹¤×÷Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖK£¬“ĖŹ±A”¢BĮ½¼«ÉĻ²śÉśµÄĘųĢåĢå»żĻąĶ¬£®
ČēĶ¼ŹĒ¼×“¼£ØCH3OH£©Č¼ĮĻµē³Ų¹¤×÷µÄŹ¾ŅāĶ¼£¬ĘäÖŠA”¢B”¢D¾łĪŖŹÆÄ«µē¼«£¬CĪŖĶµē¼«£®¹¤×÷Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖK£¬“ĖŹ±A”¢BĮ½¼«ÉĻ²śÉśµÄĘųĢåĢå»żĻąĶ¬£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 mol HClOČÜÓŚĖ®µēĄė³öNAøöH+ | |
| B£® | ±ź×¼×“æöĻĀ£¬11.2LµÄCCl4ÖŠŗ¬ÓŠ0.5 NAøö·Ö×Ó | |
| C£® | ×ćĮæFeÓė1 mol Cl2³ä·Ö·“Ó¦£¬×ŖŅʵē×ÓŹżĪŖ2NA | |
| D£® | 18.0 gÖŲĖ®£ØD2O£©ÖŠĖłŗ¬µÄµē×ÓŹżĪŖ10 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 mol¼×»ł£Ø-CH3£©Ėłŗ¬µÄµē×Ó×ÜŹżĪŖ9NA | |
| B£® | 1mol¼×Ķé×ī¶ąÓė2molCl2·¢ÉśČ”“ś·“Ó¦ | |
| C£® | ±ź×¼×“æöĻĀ£¬2.24LŅŅ“¼ŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖ0.1NA | |
| D£® | 0.5 mol±½·Ö×ÓÖŠŗ¬ÓŠC=CĖ«¼üŹżĪŖ1.5 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬2.24L±½ŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ0.1NA | |
| B£® | ³£ĪĀ³£Ń¹ĻĀ£¬18g H2Oŗ¬ÓŠ10nAøöµē×Ó | |
| C£® | 1L1mol•L-1µÄNH4ClČÜŅŗÖŠÓŠnAøöNH4+ | |
| D£® | Ņ»¶ØĢõ¼žĻĀ£¬ĻņĆܱÕČŻĘ÷ÖŠĶ¶Čė3mol H2ŗĶ1mol N2³ä·Ö·“Ó¦ŗóæɵƵ½NH3·Ö×ÓŹżĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com