
| A�������£������ʵ���Ũ�ȵ�������Һ�٣�NH4��2CO3��NH4Cl�ۣ�NH4��2Fe��SO4��2��c��NH4+�����٣��ڣ��� |
| B�������ʵ���Ũ�ȵ�H2S��NaHS�����Һ�У�c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� |
| C�������£���c��H+��=1.0��10-13mol?L-1����Һ�У�Na+��S2-��AlO2-��SO32-�����Ӳ����ܴ������� |
| D��һ���¶��£�������μ����������Һ���������ԣ���ʱ��Һ�У�c��Na+����c��Cl-��=c��CH3COOH�� |


 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������������뷢չ����Ҫ���ʻ�������ʱ����������Ҫ��־��
�������������������뷢չ����Ҫ���ʻ�������ʱ����������Ҫ��־���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
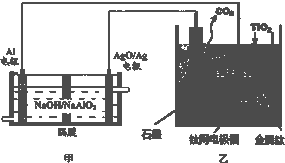
| A����װ�ù���������OH-��AgO/Ag���ƶ�����װ�ù���������O2-�������ƶ� | ||
| B����װ�������ĵ缫��ӦʽΪC+2O2--4e-�TCO2�� | ||
C������������Ч��Ϊ�ǣ����ȡ1mol Tiʱ����Al�����ʵ���
| ||
| D�����Ʊ�������ǰ������װ����CaO���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ѿ�����ڻ�ԭ�Ͷ��� |
B��һ���ܻ����ĽṹΪ ��������������֬ ��������������֬ |
| C�������������Ի���������ᡢ�Ӧ������ |
| D���ý��ݹ����������Һ�Ĺ�������ˮ���ͷų�����ϩ���ɴﵽˮ�����ʵ�Ŀ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʽΪC4H9Cl���칹����ĿΪ4 |
| B������ʽΪC8H10���칹����ĿΪ4 |
| C������ʽΪC5H12O�Ĵ����칹����ĿΪ8 |
| D������ʽΪC6H12O2�������칹����ĿΪ20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��H+��=1��10-14mol/L����Һ��K+��Cu2+��I-��SO42- |
| B��ˮ�������c��H+��=1��10-14mol/L����Һ��K+��Na+��AlO2-��S2O32- |
| C������Al��Ӧ����H2����Һ��NH4+��Ca2+��NO3-��I- |
| D������K3[Fe��CN��6]������ɫ��������Һ��H+��Na+��SO42-��CrO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��a��b��c��d |
| B��b��a��d��c |
| C��c��b��a��d |
| D��b��a��c��d |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com