
ij��ѧ��ȤС������ʵ����̽�����������ʼ�ģ�ҵ��ȡƯ�ۣ����������װ�ý���ʵ�飨ʵ������ȡ�����ķ�ӦΪMnO2 + 4 HCI(Ũ�� MnCl2 + C12��+ 2 H2O����
MnCl2 + C12��+ 2 H2O����
�밴Ҫ��ش��������⣺
��1�����۵⻯����Һ�й۲쵽��������_________����Ӧ�����ӷ���ʽ____________��
��2�����������ɫ��������ɫ��ʪ�����ɫ������ɫ�������ʢ��________�������ƣ���
��3��C12��ʯ���鷴Ӧ��ȡƯ�۵Ļ�ѧ����ʽΪ___________________��
��4������ȤС����8.7g MnO2��������Ũ�����Ʊ��������������������Ƶñ�״���µ�Cl2______________L��
��1����Һ����ɫ Cl2+2I-=2Cl-+I2
��2��Ũ����
��3��2Cl2+2Ca(OH)2��CaCl2+Ca(ClO)2+2H2O
��4��2.24
���������������1��MnO2��Ũ�����ϼ��ȷ�����Ӧ��ȡCl2,����HCl�лӷ��ԣ�ʵ����Cl2�к�������HCl���ñ���NaCl��Һ����ȥHCl�����п��Լ���Cl2�����ġ�����Cl2�������ԣ�����KI������Ӧ��Cl2+2KI=2KCl+I2��I2�����۱���ɫ����Ӧ�����ӷ���ʽΪCl2+2I-=2Cl-+I2����2�����������ɫ��������ɫ��ʪ�����ɫ������ɫ�������ʢ��Ũ���ᡣ��3��C12��ʯ���鷴Ӧ��ȡƯ�۵Ļ�ѧ����ʽΪ2Cl2+2Ca(OH)2��CaCl2+Ca(ClO)2+2H2O����4��n(MnO2)=8.7g��87g/mol="0.1mol." ����HCl���������Էų���������MnO2���������ݷ���ʽMnO2 + 4 HCI(Ũ�� MnCl2 + C12��+ 2 H2O�ɵ÷ų���C12�����ʵ���Ϊ0.1mol.�ڱ�״���£������Ϊ2.24L��
MnCl2 + C12��+ 2 H2O�ɵ÷ų���C12�����ʵ���Ϊ0.1mol.�ڱ�״���£������Ϊ2.24L��
���㣺����C12��ʵ�����Ʒ����йص����ʡ������й�����Ħ������ļ����֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ��һ�ֵij��õĻ�ѧ�Լ���
(1)��Ũ��ˮ����ʯ��Ϊԭ�ϣ�������װ��Ϊ����װ�ã�����ȡ�� ����������֪��NH3��H2O+CaO=Ca(OH)2+NH3����
����ʯ��Ӧ��װ�� (��A��B)��
���Դӵ���ƽ��Ƕȷ�����ʵ���а����ݳ���ԭ�� ��
����Ҫ���Թܺͱ�Ҫʵ����Ʒ�ռ�һ�Թܰ���������C��������Ӧ�ռ�װ�á�
(2)��ѧ��ȤС���ð�ˮ����������Һ���ƺ�������Һ������ȩ��������Ӧʵ�飬������ͬѧʵ��ʱ�䳤����Ч�������ԡ�Ӱ����ȩ������Ӧ���ʵ���������Щ�أ�����������²��룺
�ٲ��룺����һ��������Һ��pH��С��
���ض��� ��
�������ʵ����֤����Ӱ������һ������±������ݡ�
�ṩ�Լ�����ȩ��2%ϡ��ˮ�� 0��25mol/L NaOH��Һ������ �� 2% AgNO3��Һ
ʵ����Ʒ���ձ�(װ����ˮ)���Թ�
| ʵ�鲽�� | ʵ����� | Ԥ��������(����1ֻ������) |
| 1 | ��A��B��֧�ྻ���Թ��и���1mL 2%��AgNO3��Һ��Ȼ������Թܱ���ε���2%ϡ��ˮ������������ij���ǡ����ȫ�ܽ⣬��ʱ�Ƶ�pH��ԼΪ8��������Һ�� | |
| 2 | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С����ʵ�����������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣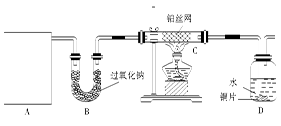
(1)A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������ (��ѡ����)����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ (ѡ������������ţ��̶�װ��ʡ��)��
(2)��װ�ò�����������Ȼ����һ����ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
�� ��
�� ��
(3)���ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B�������� ��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ�� ��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧʵ����ȤС��Ϊ����֤��ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��Cl2��ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ��(֧���õ�����̨ʡ��)���밴Ҫ��ش��������⡣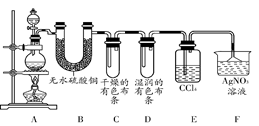
(1)���з����У����Ƶ�Cl2����ȷ����� ��
��MnO2��Ũ�����Ϲ��ȣ���MnO2��NaCl��Ũ�����Ϲ��ȣ���NaClO��Ũ�����ϣ���K2Cr2O7��Ũ�����ϣ���KClO3��Ũ�����Ϲ��ȣ���KMnO4��Ũ�����ϡ�
A���٢ڢ� B���ڢܢ�
C���٢ܢ� D��ȫ������
(2)д��ʵ������ȡCl2�����ӷ���ʽ ��
(3)��װ��B�������� ��
��װ��C��D���ֵIJ�ͬ����˵���������� ��
��װ��E�������� ��
(4)��ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣���ͬѧ���Ӧ����װ�� �� ֮��(��װ����ĸ���)����һ��װ�ã�������װ��������Լ�����Ϊ (����ĸ���)��
A��ʪ��ĵ⻯�ص�����ֽ B��Ũ����
C��ʪ��ĺ�ɫ���� D������ʳ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ͼװ�ý���ʵ�飬��֪��Na2O2��H2O��CO2���ܷ�Ӧ������O2,����NH3����Ӧ
�ش��������⣺��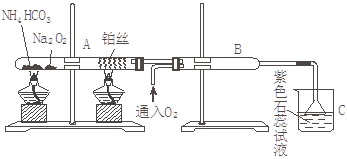
��1�������ȵ��Թ�A��NH4HCO3������Ӧ�Ļ�ѧ����ʽΪ�� ��
��2�������ȵIJ�˿�������Ļ�ѧ����ʽΪ��___________________________________��
��3��B�г��ֵ�����Ϊ��___________________________________________________��
��4���ձ�C�з���������Ϊ________________________________________________��
��5������©�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
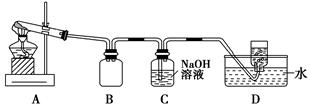
ij��ѧ����С��ͨ��ʵ��̽��NO2�����ʡ���֪��2NO2��2NaOH=NaNO3��NaNO2��H2O��
����1��������ͼ��ʾװ��̽��NO2�ܷ�NH3��ԭ(K1��K2��Ϊֹˮ�У��г�װ������ȥ)��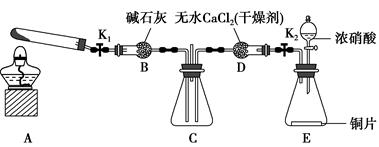
(1)Eװ������ȡNO2�Ļ�ѧ��Ӧ����ʽ��____________________________
____________________________________________��
(2)��NO2�ܹ���NH3��ԭ��Ԥ����Cװ���й۲쵽��������________________________________________________________________��
(3)ʵ������У�δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ�ӷ�Ӧԭ���ĽǶȷ�����ԭ����Ϊ���������ֿ��ܣ�
��NH3��ԭ�Խ��������ܽ�NO2��ԭ��
���ڴ������£�NO2��ת���ʼ��ͣ�
��______________________________________________________________��
(4)��ʵ��װ����һ�����Ե�ȱ����__________________________________��
����2��̽��NO2�ܷ���Na2O2����������ԭ��Ӧ��
(5)ʵ��ǰ����С��ͬѧ������ּ��衣
����1�����߲���Ӧ��
����2��NO2�ܱ�Na2O2������
����3��________________________________________________________��
(6)Ϊ����֤����2����С��ͬѧѡ������1�е�B��D��Eװ�ã���B�е�ҩƷ������Na2O2����ѡFװ��(��ͼ��ʾ)��������װ����ʵ�顣
��װ�õĺ�������˳����(ijЩװ�ÿ����ظ�ʹ��)________��
��ʵ������У�Bװ���еķ�ĩ�ɵ���ɫ��ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɡ��Ʋ�Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��AgNO3�������Ժ����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣
��.AgNO3��������
����������˿����AgNO3��Һ�У�һ��ʱ�����˿ȡ����Ϊ������Һ��Fe�������������Һ�е�Ag����������������ʵ�飬��ѡ���Լ���KSCN��Һ��K3[Fe(CN)6]��Һ����ˮ��
(1)������±���
| ���� | ���� | ���� |
| ȡ��������Ag�������Һ���Թ��У�����KSCN��Һ���� | | ����Fe3�� |
| ȡ��������Ag�������Һ���Թ��У�����________���� | | ����Fe2�� |
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ����������MnO2��Ũ���ᷴӦ��ȡ��������װ����ͼ1��ʾ��

ͼ1 ͼ2
��1��ͼ1������a�������ǣ� ������b�������ǣ� ��b�м������Ƭ�������ǣ� ��
��2����д������b�з����ķ�Ӧ�����ӷ���ʽ��
��3�����װ�õ�������֮��IJ��������ǣ� �� �� ��������ţ�
A������ƿ�м���MnO2��ĩ
B������
C������ƿ�м���Ũ����
��4���÷�Ӧ����Ϊ����Ũ���½���ֹͣ��Ϊ�˲ⶨ��Ӧ����Һ�������Ũ�ȣ�ij̽��С���������ʵ�鷽����
�ټ�ͬѧ�ķ���Ϊ��������AgNO3��Һ��Ӧ���������ɳ�����������
����ͬѧ�ķ���Ϊ����������п��Ӧ��������������������ʵ��װ����ͼ2��ʾ���г�װ������ȥ����ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ������ ����п��ת�Ƶ�������Һ�С�������Һת�Ƶ�п���С���������ȷ��ȡ�����ܶ���ʱ������Ҫƽ�ӣ�Ҫע��ʹ©��Һ������������Һ����ƽ���������ע�⣺ ��
���ַ�������Ϊ ������ң�ͬѧ�ķ������С�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com