
(1)0.3 mol NH3������������������________��H2O������������������ȡ�
(2)��0.4 mol Al3����Al2(SO4)3��������SO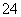 �����ʵ�����____
�����ʵ�����____ ____��
____��
(3)��֪16 g A��20 g Bǡ����ȫ��Ӧ����0.04 mol C��31.76 g D����C��Ħ������Ϊ________��
(4)2.3 g Na�к�________ mol e�����ڸ�����ˮ��Ӧ��ʧȥ________ mol e����
(5)���a gij�����к��еķ�����Ϊb����c g�������ڱ�״���µ������(��NAΪ�����ӵ�����)________��
������0.3 mol NH3�����к�������Ϊ0.3 mol��10��6.02��1023 mol��1��1.806��1024��ÿ��H2O��������10�����ӣ��ʺ�1.806��1024�����ӵ�ˮ������Ϊ1.806��1024��10��1.806��1023��Al2(SO4)3��n(Al3��)��n(SO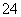 )��2��3����0.4 mol��n(SO
)��2��3����0.4 mol��n(SO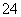 )��2��3��n(SO
)��2��3��n(SO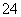 )��0.6 mol�����������غ㶨��m(C)��16 g��20 g��31.76 g��4.24 g��M(C)��
)��0.6 mol�����������غ㶨��m(C)��16 g��20 g��31.76 g��4.24 g��M(C)��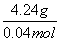 ��106 g/mol��2.3 g Na������
��106 g/mol��2.3 g Na������ ��11��1.1 mol����ˮ��Ӧʧȥ����
��11��1.1 mol����ˮ��Ӧʧȥ���� ��1��0.1 mol��a g��������ʵ���Ϊ
��1��0.1 mol��a g��������ʵ���Ϊ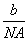 mol��������Ħ������Ϊ
mol��������Ħ������Ϊ g/mol����c g������Ϊ
g/mol����c g������Ϊ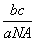 mol�����Ϊ
mol�����Ϊ L��
L��
�𰸣�(1)1.806��1023��(2)0.6 mol��(3)106 g/mol
(4)1.1��0.1��(5) L
L


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ը��������Һ�����һ�������� �� ��
A����ϩ ��Ȳ B���� ���� C������ ������ D���� �ױ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮż����Ӧ��һ�����͵�ֱ���������Ӧ�����磺
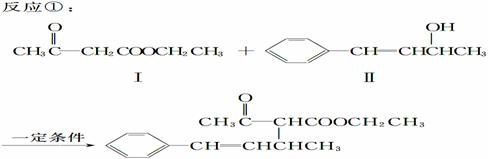
(1)���������ķ���ʽΪ________��
1 mol��������ȫȼ��������Ҫ����________ mol O2��
(2)����������ʹ________��Һ(��дһ��)��ɫ��
�������(����ʽΪC10H11Cl)����NaOHˮ��Һ�������ɻ������
��Ӧ�Ļ�ѧ����ʽΪ_____ ____��
(3)�����������NaOH�Ҵ���Һ�������ɻ�����������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2�����Ľṹ��ʽΪ__________��
(4)����CH3COOCH2CH3�ɺϳɻ�������������CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ��������
���Ľṹ��ʽΪ__________�����Ľṹ��ʽΪ______________��
(5)��һ�������£� ��
�� 
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��Z�Ƕ�����Ԫ�أ��䲿������
���ұ�������˵����ȷ����
A����̬�⻯������ȶ��ԣ�X<W
B������������Ӧˮ��������ԣ�Y��X
C�����Ӱ뾶��Z��W
D��Y���������к��зǼ��Թ��ۼ�
| W | �����ǵ���ɫ���� |
| X | �ڵؿ��еĺ����ӵڶ�λ |
| Y | ԭ�������������ǵ���������2/3 |
| Z | ��������ԭ�Ӱ뾶��С�Ľ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A��Ħ���������ʵ�������λ�������ʵ�������λ
B�������ӵ�������12 kg 12C�к��е�̼ԭ����
C��1 molˮ������ ����2 mol��ԭ�Ӻ�1 mol��ԭ��
����2 mol��ԭ�Ӻ�1 mol��ԭ��
D��һ��NO���ӵ�������a g��һ��NO2���ӵ�������b g������ԭ�ӵ�Ħ��������(b��a) g��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�������ֵ������˵����ȷ����(����)
A����״���£�22.4 L���ȼ���ķ�����ԼΪNA��
B��ʢ��SO2���ܱ������к���NA����ԭ�ӣ���SO2�����ʵ���Ϊ0.5 mol
C��17.6 g�����������ļ��Թ��ۼ�Ϊ4NA��
D����⾫��ͭʱ���������õ�������Ϊ2NA������������������64 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijҺ�廯����X2Y4�����������ȼ�ϡ�16 g X2Y4��һ������O2��ǡ����ȫȼ�գ���Ӧ����ʽΪX2Y4(l)��O2(g)===X2(g)��2Y2O(l)����ȴ���״���²������������Ϊ11.2 L����
(1)��ӦǰO2�����V(O2)Ϊ________��
(2)X2��Ħ������Ϊ________��YԪ�ص�������________��
(3)����Ӧ����0.1 mol X2����ת�Ƶ��ӵ����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��Z 4�ֶ�����Ԫ����Ԫ�����ڱ��е�λ������ͼ��ʾ������Zλ�ڢ�A�塣�����жϴ������(����)
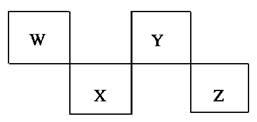
A����ѹ������Ԫ�صĵ����У�W���ʵ��۵����
B��Z�������ӵ��Ӳ�ṹ���ԭ�ӵ���ͬ
C��W���⻯��ķе��Y���⻯��ķе��
D��YԪ�صķǽ����Ա�XԪ�صķǽ�����ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A���������ǻ�״�ṹ�������ʸ�����������
B�� ��ʾ���ķ��ӽṹ�����к���̼̼˫������˱������ʸ�ϩ����ͬ
��ʾ���ķ��ӽṹ�����к���̼̼˫������˱������ʸ�ϩ����ͬ
C�����ķ���ʽΪC6H6�������е�̼ԭ��û�дﵽ���ͣ������ʹ��ˮ��ɫ
D��������ʹKMnO4������Һ��ɫ��������ͬϵ��ȴ��ʹKMnO4������Һ��ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com