



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | -- | -- | 4.7 | -- |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8 | 6.7 | 9 ����9�ܽ⣩ |
| +6 |
| Cr |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
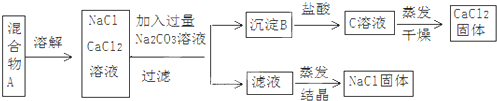
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��MnO4- Cl- NH4+ Na+ |
| B��CO32- NO3- Ca2+Na+ |
| C��SO42- HCO3- K+ Na+ |
| D��SO42-NO3-K+ Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+��Cu2+��Cl-��SO42- |
| B��Na+��H+��NO3-��CO32- |
| C��Na+��H+��OH-��SO42- |
| D��Ba2+��Cu2+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | 0.1032mol/L HCl��Һ���/mL | ����NaOH��Һ���/mL |
| 1 | 27.83 | 25.00 |
| 2 | 26.53 | 25.00 |
| 3 | 27.85 | 25.00 |
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��10-17 | 2.0��10-48 | 1.8��10-10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ǿ�������Һһ�������������Һ��������ǿ |
| B������������������� |
| C��ǿ����ʵ�ˮ��Һ�в��������ʷ��� |
| D����Ϊ������������ʣ�������ǿ����ʣ�����к͵���������ʵ���Ũ�ȵĴ��������ʱ���к��������ĵ��������Ʊ��кʹ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ֽһ�˽�����Һ���۲���ɫ�仯 |
| B������ˮ��pH��ֽ��ʪ���ٲⶨ��Һ��pH |
| C������ֽһ������Һ�н�һ��ʱ��������ɫ������ |
| D���ýྻ�IJ�����պȡ����Һ������pH��ֽ�в���Ȼ�����ɫ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com