
Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߣ�������Ԫ�����ڱ���һ���֣�����������ĸA��D��E��G��Q��M��R��T�ֱ����ijһ��ѧԪ�ء���������Ԫ�ػش��������⡣
| A | |||||||||||||||||
| D | E | ||||||||||||||||
| G | Q | M | R | ||||||||||||||
| T | |||||||||||||||||
��1��ijԪ��ԭ�ӵĺ�����Ӳ�����������������3������Ԫ�ص�ԭ�ӽṹʾ��ͼΪ ��
![]() ��2��ijЩԪ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ� ��
��2��ijЩԪ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ� ��
��3��M��D����Ԫ���γɵĻ����ﺬ�еĻ�ѧ�������� ��������ǣ�����ԡ��Ǽ��ԡ��� ���ӣ�A�ֱ���D��E��R�γɵķ����У����Ӽ����������ǣ������ʽ�� ��A��D�γɷ��ӵĿռ�ṹ�����ǣ�����ţ� ��
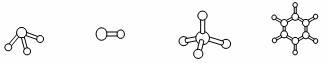
a b c d
ͼ1
��4��������Ԫ������ɺ��ʵķ�Ӧ��û�ѧ����ʽ����Ԫ��M��R�ķǽ�����ǿ����
��
��5��������������Ԫ�ص����۵�ߵ͵�˳����ͼ2��ʾ����1�����������ʵĻ�ѧʽ�� ����7������Ӧ��Ԫ���γ����������ľ��������� ��

ͼ2
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | ||||||||||||||||||
| M | B | D | ||||||||||||||||
| G | H | Q | R | |||||||||||||||
| E | ||||||||||||||||||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





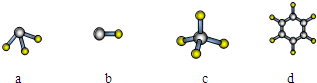
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߡ��±���Ԫ�����ڱ���һ���֣�����������ĸA��B��D��E��G��H��Q��M��R�ֱ����ijһ��ѧԪ�ء���������Ԫ�ػش��������⡣
(1)QԪ����Ԫ�����ڱ��е�λ�� ��EԪ��ԭ�ӽṹʾ��ͼΪ_____
(2)E��Q��R����Ԫ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ�_________________________________��
(3)M��D��Ԫ���γɵĻ������к��еĻ�ѧ�������� ����MD2���ӵĽṹʽ�� ��
(4) �ñ�������Ԫ����ɷ�Ӧ��û�ѧ����ʽ˵��Ԫ��Q��R�ǽ����Ե�ǿ����
��Ԫ��G��H�Ľ�����ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ϻ���բ����������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߣ�Ԫ�������ɷ�ӳ��Ԫ�����ʵ������Ա仯���ɡ�
��1��Ԫ��A��ԭ�����������Ų�ʽΪnsnnpn����ԭ������㹲�� �ֲ�ͬ�˶�״̬�ĵ��ӣ���ԭ�Ӻ���� ��������ͬ�ĵ��ӡ�Ԫ��A����Ԫ���γɵķ��ӣ���ռ�ṹ�����ǣ�����ţ� ��
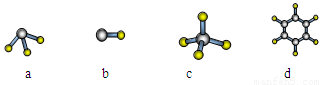
��2�����б仯��������ȷ���� ��
A������K��Na��Mg���۷е��ɵ͵���
B��C1-��Br-��I-��ʧ��������������ǿ
C��H+��Li+��H-�İ뾶�ɴ�С
D��H3PO4��HClO4��H2SO4������������ǿ
��3���ڶ�����Ԫ���У���Be��B��Ne����Ԫ���⣬����Ԫ�ص��⻯��е����±���ʾ������A�ĵ���ʽΪ ��E�Ļ�ѧʽΪ ��
|
�⻯�� |
A |
B |
HF |
D |
E |
|
�е�/�� |
1317 |
100 |
19.5 |
33 |
164 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��һ�ڶ�ѧ����ĩ��ϰ��ѧ�Ծ�1 ���ͣ������
��10�֣�Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߡ��±���Ԫ�����ڱ���һ���֣�����������ĸA��B��D��E��G��H��Q��M��R�ֱ����ijһ��ѧԪ�ء���������Ԫ���ش��������⡣
(1)QԪ����Ԫ�����ڱ��е�λ�� ��EԪ��ԭ�ӽṹʾ��ͼΪ_____
(2)E��Q��R����Ԫ�ص�ԭ�ӿ��γ���Ar������ͬ���Ӳ�ṹ�ļ����ӣ���Щ���ӵİ뾶�ɴ�С��˳���ǣ������ӷ��ţ�_________________________________��
(3)M��D��Ԫ���γɵĻ������к��еĻ�ѧ�������� ����MD2���ӵĽṹʽ�� ��
(4) �ñ�������Ԫ����ɷ�Ӧ��û�ѧ����ʽ˵��Ԫ��Q��R�ǽ����Ե�ǿ����
��Ԫ��G��H�Ľ�����ǿ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com