
��1��д��D�ķ���ʽ____________________��G�Ľṹ��ʽ________________��
��2��д�����л�ѧ��Ӧ����ʽ��ע����Ӧ����
��Ӧ��___________________________��____________��
��Ӧ��___________________________��____________��
��Ӧ��___________________________��____________��
��3��д�����ַ���������������D��ͬ���칹��Ľṹ��ʽ��������������ȡ�������ڶ�λ���������ࣩ��____________________________��_____________________________��
��4���Լ���������У�ÿ����1.00�ָ߷��ӻ��������������NaOH����_______�֡�
��5����ɫ��ѧ��������ԭ����ԭ�ӣ�ʵ�����ŷŻ����ŷš��������д�������NaOH��̸һ̸��Ľ�������룺___________________________________________________________��

��Ӧ�ݣ�BrCH2CH2Br+2NaOH![]() HOCH2CH2OH+2NaBr ȡ����ˮ�⣩
HOCH2CH2OH+2NaBr ȡ����ˮ�⣩
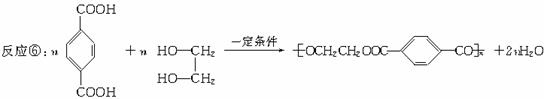 �ۺϣ����ۣ�
�ۺϣ����ۣ�
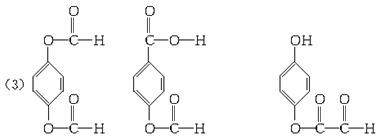
��4��0.833
��5���ɸ����ȼ�գ�������NaCl��Һ��⣺
2NaCl+2H2O![]() 2NaOH+H2��+Cl2
2NaOH+H2��+Cl2
����NaOHѭ�������л��ϳɣ�����Cl2���û���Br2��
Cl2+2NaBr====2NaCl+Br2
ѭ�������л��ϳɣ�H2�ռ�������Ʒ���ۡ������������ɵ÷֣�
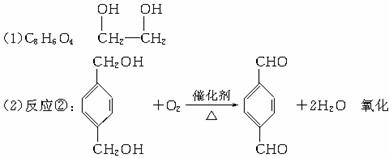
������ͼ�У���B��������������ΪD��DΪ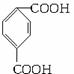 ���ɸ߷��ӻ�����Ľṹ��ʽ֪GΪHOCH2CH2OH��AΪ
���ɸ߷��ӻ�����Ľṹ��ʽ֪GΪHOCH2CH2OH��AΪ![]() ��BΪ
��BΪ![]() ��FΪBrCH2CH2Br��
��FΪBrCH2CH2Br��
��Ӧ��Ϊ![]() ��Ӧ��ΪBrCH2CH2Br+2NaOH
��Ӧ��ΪBrCH2CH2Br+2NaOH![]() HOCH2CH2OH+2NaBr���ɷ�Ӧ�٢ݢ�
HOCH2CH2OH+2NaBr���ɷ�Ӧ�٢ݢ�
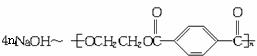
160 192
![]() 1 t
1 t
���ÿ����1.00�ָ߷��ӻ��������������NaOH����![]() =0.833 t��
=0.833 t��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������RCOOR���ڴ�������ʱ�ɸ�R��OH���������·�Ӧ(R�䣬R�������ֲ�ͬ������)��RCOOR��+R��OH![]() RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��У��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ��
RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��У��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ�� ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�
ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�
������������⣺
(1)��Ӧ�м״�ΪʲôҪ������__________________________________________��
(2)д������ϩ���Ľṹ��ʽ��_____________________________________________��
(3)д����������ϩ����״�������������Ӧ�Ļ�ѧ����ʽ��__________________________��
��ˮ�ⷴӦ���ƣ���һ�������£���Ҳ�ܷ������ⷴӦ����д�����з�Ӧ����ʽ��
(4)��������ϩ����ˮ�⣺______________________________________��
(5)��������ϩ���İ��⣺______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ���ϳɷ�Ӧ������ͼ��A��G��Ϊ�л����Ӧ�в����������ʡ�ԣ���һ����Ӧ�е��Լ��������е���ע�����е�δע��������ͼ��գ�
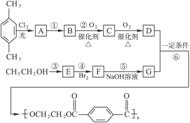
��1��д��D�ķ���ʽ____________________��G�Ľṹ��ʽ________________��
��2��д�����л�ѧ��Ӧ����ʽ��ע����Ӧ����
��Ӧ��___________________________��____________��
��Ӧ��___________________________��____________��
��Ӧ��___________________________��____________��
��3��д�����ַ���������������D��ͬ���칹��Ľṹ��ʽ��������������ȡ�������ڶ�λ���������ࣩ��
_____________________________��_____________________________��
��4���Լ���������У�ÿ����1.00�ָ߷��ӻ��������������NaOH����_______�֡�
��5����ɫ��ѧ��������ԭ����ԭ�ӣ�ʵ�����ŷŻ����ŷš��������д�������NaOH��̸һ̸��Ľ�������룺
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ϩ���������ϳ���ά����ά�ڡ��ϼ�������ˮ��Ϳ�ϡ�Ӥ����ʪ���ϵ�һ����Ҫ����ԭ�ϡ�����ϩ�������ˮ���ԣ����ͷ��л��ܼ���Ⱦ�������������ǵ�ϲ������������������ϩ����һ�ַ�Ӧԭ����
������RCOOR���ڴ�������ʱ�ɸ�R��OH���������·�Ӧ(R�䣬R�������ֲ�ͬ������)��RCOOR��+R��OH![]() RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��У��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ��
RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��У��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ�� ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�
ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�
������������⣺
(1)��Ӧ�м״�ΪʲôҪ������__________________________________________________��
(2)д������ϩ���Ľṹ��ʽ��____________________________________________________��
(3)д����������ϩ����״�������������Ӧ�Ļ�ѧ����ʽ��__________________________��
��ˮ�ⷴӦ���ƣ���һ�������£���Ҳ�ܷ������ⷴӦ����д�����з�Ӧ����ʽ����ָ����Ӧ���͡�
(4)��������ϩ����ˮ�⣺_________________��______________________________________��
(5)��������ϩ���İ��⣺_________________��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ϸ߶��и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
��ÿһС��2�ֹ�12�֣�������һ���ϳɷ�Ӧ������ͼ��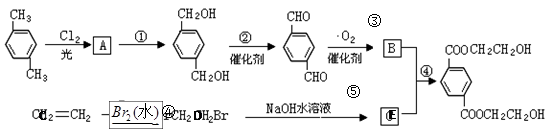
��1����д���������ʵĽṹ��ʽ
| A���� | B���� |
| C���� | D�� . |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com