




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����16�֣�
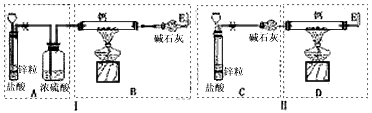
��֪���������������������壩��H2�ڸ�����������ʱҪ�Է�����Ӧ�����������ͽ����⻯���NaH��CaH2�ȣ���������ˮ��Ӧ�����мס�����λͬѧ�ֱ�������Ʊ�CaH2��ʵ�飬װ����ͼ��ʾ���ֱ�����Ţ��ʾ��.
��ش���������
��1��п�����ᷴӦ�����ӷ���ʽΪ ��
��2�����ʵ������ʾ����λͬѧ��ʵ��װ����ƶ���ȱ�ݡ�
װ�â�IJ���֮���� ��
װ�â�IJ���֮���� ��
��3�����������װ����ѡȡ����Ϊ�����IJ��֣��������ҵ�˳����װһ����ȡCaH2�ĺ���װ�ã������A��B��C������ʾ�� ��
��4�������װ�õ������Ժ�Ϊ�˱�֤��Ʒ�Ĵ��Ⱥ�ʵ�鰲ȫ������ ��Ȼ����
�����ܵ�ȼ�ƾ��Ƽ��ȡ�ͬ����Ϊ�˰�ȫ����Ӧ��ʼ����E�ڴ�Ӧ ��
��5�����º�����Ұ����ҵ����CaH2���������ʹ֮��ˮ��Ӧ����H2���䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ݷ��ʵ�����2010�������ǰȫ��ģ�⣨���ۻ�ѧ���֣� ���ͣ�ʵ����
�����16�֣�
��֪���������������������壩��H2�ڸ�����������ʱҪ�Է�����Ӧ�����������ͽ����⻯���NaH��CaH2�ȣ���������ˮ��Ӧ�����мס�����λͬѧ�ֱ�������Ʊ�CaH2��ʵ�飬װ����ͼ��ʾ���ֱ�����Ţ��ʾ��.

��ش���������
��1��п�����ᷴӦ�����ӷ���ʽΪ ��
��2�����ʵ������ʾ����λͬѧ��ʵ��װ����ƶ���ȱ�ݡ�
װ�â�IJ���֮���� ��
װ�â�IJ���֮���� ��
��3�����������װ����ѡȡ����Ϊ�����IJ��֣��������ҵ�˳����װһ����ȡCaH2�ĺ���װ�ã������A��B��C������ʾ�� ��
��4�������װ�õ������Ժ�Ϊ�˱�֤��Ʒ�Ĵ��Ⱥ�ʵ�鰲ȫ������ ��Ȼ����
�����ܵ�ȼ�ƾ��Ƽ��ȡ�ͬ����Ϊ�˰�ȫ����Ӧ��ʼ����E�ڴ�Ӧ ��
��5�����º�����Ұ����ҵ����CaH2���������ʹ֮��ˮ��Ӧ����H2���䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 ģ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com