
���ú�ˮ������ȡ���þ����ȡ�������¡�
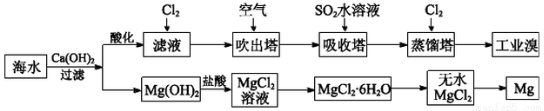
(1)��ȡ��Ĺ����У���������Br����Br2ת����Ŀ����________���������з�����Ӧ�����ӷ���ʽ��__________����ƽ���ƶ�ԭ������ͨ��������ҪĿ����_______��
(2)��MgCl2��Һ�еõ�MgCl2��6H2O�������Ҫ������________________�����ˡ�ϴ�ӡ����
(3)�����������̣�����10 m3��ˮ�е���Ԫ��ת��Ϊ��ҵ�壬������Ҫ��״����Cl2�����Ϊ________L(����Cl2���ܽ�)��
(1)����Ԫ�ؽ��и�����SO2��Br2��2H2O=4H����2Br����SO42����ͨ�������������������ʹBr2(aq) Br2(g)ƽ�������ƶ���(2)����Ũ������ȴ�ᾧ
Br2(g)ƽ�������ƶ���(2)����Ũ������ȴ�ᾧ
(3)179.2
��������(2)���ڵõ�����MgCl2��6H2O��������Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ��裬������ֱ�ӽ���Һ���ɡ�(3)10 m3��ˮ��Br��������Ϊ104L��64��10��3 g��L��1��640 g������Cl2��2Br��=2Cl����Br2����֪һ��ת������89.6 L Cl2(��״����)��������Ҫ179.2 L Cl2(��״����)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 ģ��ˮƽ���1��ϰ���������棩 ���ͣ������
ij�����л���X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%������Ϊ����
��1��X�ķ���ʽ��________��
��2��X��������Ʒ�Ӧ�ų���������X�ṹ�к��еĹ����ŵ�����Ϊ________��
��3��X������е������ڴ��������·�Ӧ������Y���÷�Ӧ�Ļ�ѧ����ʽ��______________________����Ӧ����Ϊ________��
��4��X��������������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ���������£�X��Z��Ӧ������һ������ζ������W����184 g X��120 g Z��Ӧ����132 g W���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________������X��ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4�»�ѧ����Ȼ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
��ҵ����ȡ����ͭ����ֱ����Ũ������ͭ��Ӧ�����ǽ�ͭ˿����ϡ�����в����ϵش������²�����ϸС�Ŀ����ݣ����������ŵ��ǣ���������
����ʡ��Դ������������Ⱦ������SO2���������H2SO4�������ʡ��������Cu��������
A���٢� B���ۢ� C���٢ڢ� D��ȫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4.2��Դ�ۺ����û���������ϰ���������棩 ���ͣ������
��֪ú�Ľ����ṹģ����ͼ��ʾ��

�ش��������⣺
(1)��ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��____________����Ҫ��Դ��
(2)�ҹ���Լ70%��ú��ֱ������ȼ�յġ���ú�Ľṹģ�����������ṩ������ͬʱ����������____________��____________���������ʣ�������صĴ�����Ⱦ��
(3)����ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ��[��]�ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�չ����������ȶ��������Ρ�ij����ú������������ʯ��ʯ������������û�ѧ����ʽ��ʾ��������________________��________________��
(4)Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������____________��____________��____________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4.2��Դ�ۺ����û���������ϰ���������棩 ���ͣ�ѡ����
��Ԥ����Ⱦ��Ҫ���������ø����ֶμ��ٸ�����Ⱦ����ŷţ������Դ����Դ������Ч�ʣ���ɫ��ѧ����Ԥ����Ⱦ���Ļ����ֶΣ����и�����������ɫ��ѧ����(����)��
A������������ B��������Ⱦ��
C�������ж����ŷ� D���ž���ȾԴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4.1.2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
�������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ����ͼ�ǴӺ�ˮ����ȡijЩԭ�ϵ�����ͼ��
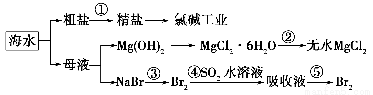
�����й�˵����ȷ����(����)��
A���������г�ȥ�����е�SO42����Ca2����Mg2����Fe3�������ʣ������ҩƷ˳��ΪNa2CO3��Һ�D��NaOH��Һ�D��BaCl2��Һ�D�����˺������
B����ҵ��ͨ���ȼҵ��ȡ������
C���ӵ���������������Ŀ����Ũ���������嵥��
D���������ķ�Ӧ�����Ǹ��¼���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4.1.2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
�������ʮ�����Ӻ�ˮ��Դ���ۺ����ã�����Ҫ��ѧ�仯���ܴӺ�ˮ�л�õ�������(����)��
A��Cl2��Br2��I2 B��Na��Mg��Al
C��ʳ�Ρ���ˮ D��NaOH��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 4.1.1��������Ŀ���������ϰ���������棩 ���ͣ�ѡ����
����˵���������(����)��
A���ԷϾɽ�������ô��������ǻ��ա�����
B����������Ҫ������ʯ�ĸ�����ұ������������
C�����ý�����ұ������ͨ�����������Һ
D���Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����2 3.3.1�Ҵ���ϰ���������棩 ���ͣ������
�Ҵ�������Ϊһ���������ȼ�ϣ���Ŀǰ�����Ͽ�������Դ�ķ�չ�ص㣬���Ҿ��нϺõľ���Ч������Ч�棬�ս���Ϊ���ͺͲ��͵����Ʒ��
(1)д���Ҵ���ȫȼ�յĻ�ѧ����ʽ��______________________________��
(2)�Ҵ�ȼ��ʱ����������㣬���ܻ���CO���ɡ�����ͼװ����֤�Ҵ���ȼ�ղ�������CO��CO2��H2O��Ӧ���Ҵ���ȼ�ղ�������ͨ��(�����������ҵ�˳����װ�ñ��)________��

(3)ʵ��ʱ�ɹ۲쵽װ������Aƿ��ʯ��ˮ����ǡ�Aƿ��Һ��������________��Bƿ��Һ��������________��Cƿ��Һ��������_________________________��
(4)װ������������________��װ��������ʢ����________��������______________________��
(5)װ��������ʢ�Ĺ���ҩƷ��________����������֤�IJ�����________��
(6)β��Ӧ��δ�����_________��
(7)�����д����ļ���ˮ����������ļ�����Ҵ���ȫȼ�ղ�����������CO2�϶����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com