
¢ń£®Ä³ČÜŅŗÖŠ½öŗ¬ĻĀ±ķĄėÓčÖŠµÄ5ÖÖĄė×ӣز»æ¼ĀĒĖ®µÄµēĄė¼°Ąė×ÓµÄĖ®½ā£©£¬ĒŅø÷Ąė×ÓµÄĪļÖŹµÄĮæ¾łĻąµČ¶¼ĪŖ0£®0lmol”£

¢ŁČōĻņŌČÜŅŗÖŠ¼ÓČėKSCNČÜŅŗ£¬ĪŽĆ÷ĻŌ±ä»Æ”£
¢ŚČōĻņŌČÜŅŗÖŠ¼ÓČė¹żĮæµÄŃĪĖį,ÓŠĘųĢåÉś³É£®ČÜŅŗÖŠŅõĄė×ÓÖÖĄą²»±ä”£
¢ŪČōĻņŌČÜŅŗÖŠ¼ÓČėBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā
£Ø1£©ČōĻČĻņŌČÜŅŗÖŠĻČ¼ÓČė¹żĮæµÄŃĪĖį£¬ŌŁ¼ÓČėKSCNČÜŅŗ£¬ĻÖĻóŹĒ__________________”£øĆ¹ż³ĢÖŠÉę¼°µÄĄė×Ó·½³ĢŹ½ÓŠ________________”¢__________________”£
£Ø2£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢×ĘÉÕ£¬×īÖÕĖłµĆ¹ĢĢåĪŖ____________________£ØŠ“»ÆѧŹ½£©”£
£Ø3£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæŃĪĖįŗó£¬ÓĆÅÅĖ®·ØŹÕ¼ÆĖł²śÉśµÄĘųĢå²¢Ź¹ĘųĢåĒ”ŗĆ³äĀśČŻĘ÷£¬½«ČŻĘ÷µ¹ÖĆÓŚĖ®²ŪÖŠ£¬ŌŁĻņČŻĘ÷ÖŠĶØČė___________mLO2£ØĘųĢåĢå»ż¾łÖø±ź×¼×“æö£©£¬ÄÜŹ¹ČÜŅŗ³äĀśøĆČŻĘ÷”£
¢ņ£®amolCu2SŗĶbmol FeSĶ¶Čėµ½v L c mol£ÆLµÄĻ”ĻõĖįÖŠ£»³ä·Ö·“Ó¦£¬Éś³ÉNOĘųĢ壬ĖłµĆ³ĪĒåČÜŅŗæÉŅŌæ“×÷Cu£ØNO3£©2”¢Fe£ØNO3£©3ŗĶH2SO4µÄ»ģŗĻČÜŅŗ£¬Ōņ·“Ó¦ÖŠĪ“±»»¹ŌµÄĻõĖįµÄĪļÖŹµÄĮæĪŖ ___________mol£ØÓĆĶ¬Ź±ŗ¬ÓŠa”¢b”¢v”¢cµÄ±ķ“ļŹ½±ķŹ¾£©”£
¢ó£®ŅŃÖŖCH4£Øg£©£«2O2£Øg£© CO2£Øg£©£«2H2O£Øl£© ”÷H1£½a kJ£ÆmolÓū¼ĘĖć·“Ó¦CH4£Øg£©£«4NO£Øg£©
CO2£Øg£©£«2H2O£Øl£© ”÷H1£½a kJ£ÆmolÓū¼ĘĖć·“Ó¦CH4£Øg£©£«4NO£Øg£© 2N2£Øg£©£«CO2£Øg£©£«2H2O£Øl£©µÄģŹ±ä”÷H2£¬Ōņ»¹ŠčŅŖ²éÕŅij·“Ó¦µÄģŹ±ä”÷H3£¬µ±·“Ó¦ÖŠø÷ĪļÖŹ»Æѧ¼ĘĮæŹżÖ®±ČĪŖ×ī¼ņÕūŹż±ČŹ±”÷H3£½b kJ£Æmol£¬ŌņøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ______________________________________”£
2N2£Øg£©£«CO2£Øg£©£«2H2O£Øl£©µÄģŹ±ä”÷H2£¬Ōņ»¹ŠčŅŖ²éÕŅij·“Ó¦µÄģŹ±ä”÷H3£¬µ±·“Ó¦ÖŠø÷ĪļÖŹ»Æѧ¼ĘĮæŹżÖ®±ČĪŖ×ī¼ņÕūŹż±ČŹ±”÷H3£½b kJ£Æmol£¬ŌņøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ______________________________________”£
¾Ż“Ė¼ĘĖć³ö”÷H2£½_____________kJ£Æmol£ØÓĆŗ¬aŗĶbµÄŹ½×Ó±ķŹ¾£©”£
I£®£Ø1£©ÓŠĘųÅŻ²śÉś£¬ČÜŅŗ±ä³É£ØŃŖ£©ŗģÉ«£Ø2·Ö£©£ØÖ»ŅŖøų³öČÜŅŗ±ä³É£ØŃŖ£©ŗģÉ«¼“µĆ·Ö£©
3Fe2++4H++NO3-=3Fe3++NO”ü+2H2O”¢Fe3++3SCN-= Fe £ØSCN£©3(Š“³ÉæÉÄę·ūŗÅøų·Ö)£Ø2·Ö£©
£Ø2£©Fe2O3”¢CuO£Ø2·Ö£©£ØĀ©Š“Ņ»ÖÖµĆŅ»·Ö£¬ÓŠ“ķŠ“²»øų·Ö£©(ĘäĖūŗĻĄķ“š°øŅ²æÉ)
£Ø3£©56£Ø2·Ö£©
¢ņ. 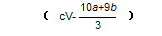 £Ø2·Ö£©
£Ø2·Ö£©
¢ó. N2(g) + O2(g)£½2NO(g) ”÷H3 = b kJ/mol £Ø2·Ö£© a - 2b£Ø2·Ö£©
»ņ2NO(g)£½N2(g) + O2(g) ”÷H3 = b kJ/mol a +2b
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗŅĄĢāŅāæÉŅŌĶĘ³öČÜŅŗÖŠŗ¬ÓŠµÄĄė×ÓÖÖĄąÓ¦ÓŠFe2+£¬NO3-
£¬Cu2+ £¬SO42-£¬Cl-”£æÉŅŌ»Ų“šĪŹĢā£Ø1£©”££Ø2£©Ó¦æ¼ĀĒFe£ØOH£©2ŌŚ¼ÓČČŹ±Éś³ÉFe£ØOH£©3£¬Cu£ØOH£©2ŗĶFe£ØOH£©3ŌŚ×ĘÉÕŹ±Éś³ÉFe2O3”¢CuO”££Ø3£©ŅņĪŖ4NO + 3O2 + 2H2O = 4HNO3,ĄūÓĆFe2+µÄĮæĪŖ0.01mol£¬æÉŅŌĖć³öO2µÄĢå»ż”£¢ņĄūÓƵē×ӵƏ§ŹŲŗćæÉŅŌĶĘ³ö±»Ńõ»ÆµÄĻõĖįĪŖ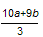 £¬ÓĆĻõĖįµÄ×ÜĮæ¼õČ„±»Ńõ»ÆµÄĻõĖįæÉŅŌĒó³ö”£¢óæÉŅŌĄūÓĆøĒĖ¹¶ØĀÉ£¬Óū¼ĘĖć·“Ó¦CH4£Øg£©£«4NO£Øg£©
£¬ÓĆĻõĖįµÄ×ÜĮæ¼õČ„±»Ńõ»ÆµÄĻõĖįæÉŅŌĒó³ö”£¢óæÉŅŌĄūÓĆøĒĖ¹¶ØĀÉ£¬Óū¼ĘĖć·“Ó¦CH4£Øg£©£«4NO£Øg£© 2N2£Øg£©£«CO2£Øg£©£«2H2O£Øl£©£¬ĪŅĆĒ»¹ŠčŅŖÖŖµĄN2ÓėNOµÄ¹ŲĻµ”£“ųČėN2(g) + O2(g)£½2NO(g) ”÷H3 = b kJ/mol£¬”÷H2=”÷H1-2”÷H3
2N2£Øg£©£«CO2£Øg£©£«2H2O£Øl£©£¬ĪŅĆĒ»¹ŠčŅŖÖŖµĄN2ÓėNOµÄ¹ŲĻµ”£“ųČėN2(g) + O2(g)£½2NO(g) ”÷H3 = b kJ/mol£¬”÷H2=”÷H1-2”÷H3
æ¼µć£ŗĄūÓĆĄė×ӵĵēŗÉŹŲŗć½ųŠŠĶʶĻ£¬²¢æ¼²ģĪļÖŹ¼ä·“Ó¦Ź±µÄµē×Ó×ŖŅĘŗĶ»Æѧ·½³ĢŹ½¼°ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“”£


 ÖĒÄÜѵĮ·Į·²āæ¼ĻµĮŠ“š°ø
ÖĒÄÜѵĮ·Į·²āæ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ½Ī÷Ź”øÓÖŻŹŠøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ijČÜŅŗÖŠ½öŗ¬ĻĀ±ķĄė×ÓÖŠµÄ5ÖÖĄė×ӣز»æ¼ĀĒĖ®µÄµēĄė¼°Ąė×ÓµÄĖ®½ā£©£¬ĒŅĄė×ÓµÄĪļÖŹµÄĮæ¾łĪŖ1mol”£
|
ŅõĄė×Ó |
SO42-ӢNO3-ӢCl- |
|
ŃōĄė×Ó |
Fe3+ӢFe2+ӢNH4+ӢCu2+ӢAl3+ |
¢ŁČōĻņŌČÜŅŗÖŠ¼ÓČėKSCNČÜŅŗ£¬ĪŽĆ÷ĻŌ±ä»Æ”£¢ŚČōĻņŌČÜŅŗÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ÓŠĘųĢåÉś³É£¬ČÜŅŗÖŠŅõĄė×ÓÖÖĄą²»±ä”£¢ŪČōĻņČÜŅŗÖŠ¼ÓČėBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³ÉŹŌ»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ČōĻČĻņŌČÜŅŗÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ŌŁ¼ÓČėKSCNČÜŅŗ£¬ĻÖĻóŹĒ ”£
£Ø2£©ŌČÜŅŗÖŠŗ¬ÓŠµÄŃōĄė×ÓŹĒ ”£
£Ø3£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæµÄŃĪĖį£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢×ĘÉÕ£¬×īÖÕĖłµĆ¹ĢĢåÓĆĶŠÅĢĢģĘ½³ĘĮæÖŹĮæĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½Ī÷Ź”ŅĖ“ŗŹŠøßČż4ŌĀÄ£Äāæ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
¢ń£®Ä³ČÜŅŗÖŠ½öŗ¬ĻĀ±ķĄė×ÓÖŠµÄ5ÖÖĄė×ӣز»æ¼ĀĒĖ®µÄµēĄė¼°Ąė×ÓµÄĖ®½ā£©£¬ĒŅĄė×ÓµÄĪļÖŹµÄĮæ¾łĪŖ1mol”£
|
ŅõĄė×Ó |
SO42-ӢNO3-ӢCl- |
|
ŃōĄė×Ó |
Fe3+ӢFe2+ӢNH4+ӢCu2+ӢAl3+ |
¢ŁČōĻņŌČÜŅŗÖŠ¼ÓČėKSCNČÜŅŗ£¬ĪŽĆ÷ĻŌ±ä»Æ”£¢ŚČōĻņŌČÜŅŗÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ÓŠĘųĢåÉś³É£¬ČÜŅŗÖŠŅõĄė×ÓÖÖĄą²»±ä”£¢ŪČōĻņŌČÜŅŗÖŠ¼ÓČėBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É”£ŹŌ»Ų“šĻĀĮŠĪŹĢā
£Ø1£©ČōĻČĻņŌČÜŅŗÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ŌŁ¼ÓČėKSCNČÜŅŗ£¬ĻÖĻóŹĒ ”£
£Ø2£©ŌČÜŅŗÖŠŗ¬ÓŠµÄŃōĄė×ÓŹĒ ”£
£Ø3£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæµÄŃĪĖį£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻņŌČÜŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢×ĘÉÕ£¬×īÖÕĖłµĆ¹ĢĢåÓĆĶŠÅĢĢģĘ½³ĘĮæÖŹĮæĪŖ ”£
¢ņ. ²ŻĖįŃĒĢś¾§Ģå£ØFeC2O4”¤2H2O£©”¢Ģ¼Ėįļ®ŗĶ¶žŃõ»Æ¹čŌŚė²ĘųÖŠøßĪĀ·“Ó¦æÉÖʱøļ®µē³ŲµÄÕż¼«²ÄĮĻ¹čĖįŃĒĢśļ®£ØLi2FeSiO4£©”£²ŻĖįŃĒĢś¾§ĢåŌŚė²ĘųĘų·ÕÖŠ½ųŠŠČČÖŲ·ÖĪö£¬½į¹ūČēÓŅĶ¼ĖłŹ¾£ØTG%±ķŹ¾²ŠĮō¹ĢĢåÖŹĮæÕ¼Ōѳʷ×ÜÖŹĮæµÄ°Ł·ÖŹż£©,Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø5£©²ŻĖįŃĒĢś¾§ĢåÖŠĢ¼ŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ£ŗ
£Ø6£©A”śB·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø7£©¾«Č·ŃŠ¾æ±ķĆ÷£¬B”śCŹµ¼ŹŹĒ·ÖĮ½²½½ųŠŠµÄ£¬ĆæŅ»²½Ö»ŹĶ·ÅŅ»ÖÖĘųĢ壬µŚ¶ž²½ŹĶ·ÅµÄĘųĢåµÄĻą¶Ō·Ö×ÓÖŹĮæ½ĻµŚŅ»²½µÄ“ó£¬ŌņµŚŅ»²½ŹĶ·ÅµÄĘųĢå»ÆѧŹ½ĪŖ£ŗ £»ŹĶ·ÅµŚ¶žÖÖĘųĢåŹ±£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ŅõĄė×Ó | SO42-”¢NO3-”¢Cl- |
| ŃōĄė×Ó | Fe3+”¢Fe2+”¢NH4+”¢Cu2+”¢Al3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÄ£ÄāĢā ĢāŠĶ£ŗŹµŃéĢā
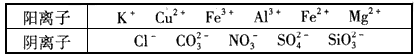
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com