
���� �ɢٵõ���ɫ��Һ����һ������CuCl2����ɫ��������Ϊ���ᱵ��̼�ᱵ�����������е�һ�֣�
�ɢڿ�֪��ҺpH��6����Һ�����ԣ���һ������Na2CO3����֪���а�ɫ��������̼�ᱵ��
�ɢ۳������μ����������������۲쵽�����������ܽ⣬��֪���а�ɫ����Ϊ���ᱵ����ԭ�������һ������NH4��2SO4��Ba��NO3��2��
�ɢ�ȡ������ʵ��٣���ɫ��Һ����μ�������������Һ���������ɹ۲쵽���г������֣����������ܽ⣬�����ȫ��ʧ�������Ϊ������������ԭ�����һ����Al2��SO4��3������������Һ��������ʹ��ɫʯ����ֽ���������壬����Ϊ������һ������NH4��2SO4��
��ȡʵ��������õ���Һ���μ���������Һ���а�ɫ�������ɣ���ɫ��������ΪAgCl�����������Դ������
��� �⣺�ɢٵõ���ɫ��Һ����һ������CuCl2����ɫ��������Ϊ���ᱵ��̼�ᱵ�����������е�һ�֣�
�ɢڿ�֪��ҺpH��6����Һ�����ԣ���һ������Na2CO3����֪���а�ɫ��������̼�ᱵ��
�ɢ۳������μ����������������۲쵽�����������ܽ⣬��֪���а�ɫ����Ϊ���ᱵ����ԭ�������һ������NH4��2SO4��Ba��NO3��2��
�ɢ�ȡ������ʵ��٣���ɫ��Һ����μ�������������Һ���������ɹ۲쵽���г������֣����������ܽ⣬�����ȫ��ʧ�������Ϊ������������ԭ�����һ����Al2��SO4��3������������Һ��������ʹ��ɫʯ����ֽ���������壬����Ϊ������һ������NH4��2SO4��
��ȡʵ��������õ���Һ���μ���������Һ���а�ɫ�������ɣ���ɫ��������ΪAgCl��������������ȷ���Ƿ�NaCl��
��1��������������ɫ������һ�����ڣ�NH4��2SO4��Al2��SO4��3��Ba��NO3��2�����ܴ���NaCl��һ��������CuCl2��Na2CO3��
�ʴ�Ϊ����NH4��2SO4��Al2��SO4��3��Ba��NO3��2��NaCl��
��2��ʵ����в���������������������ӷ���ʽ�ֱ�ΪAl3++3OH-=Al��OH��3����NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
�ʴ�Ϊ��Al3++3OH-=Al��OH��3����NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
��3�����ܴ��ڵ�������Ϊ�����ӣ�Ӧ���õIJ������۲쵽��������ȡʵ������õ���Һ�������������ԣ��μ���������Һ�������ְ�ɫ������֤����Cl-���ӣ�������Cl-���ӣ��ʴ�Ϊ��ȡʵ������õ���Һ�������������ԣ��μ���������Һ�������ְ�ɫ������֤����Cl-���ӣ�������Cl-���ӣ�
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬�������ʵ����ʡ������ķ�Ӧ������Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��Ӧ�ã���Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
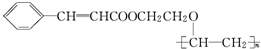

 +
+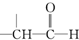 $��_{��}^{ϡOH-}$
$��_{��}^{ϡOH-}$ +H2O
+H2O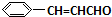 +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2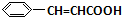 +H2O����C��F�ķ�Ӧ��
+H2O����C��F�ķ�Ӧ��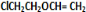 +
+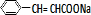 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$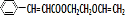 +NaCl��
+NaCl�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
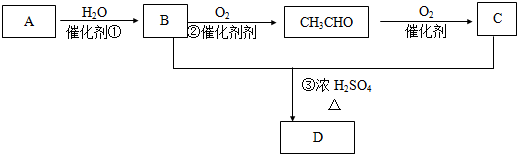
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S��I2��Fe3+ | B�� | Fe3+��I2��S | C�� | Fe3+��S��I2 | D�� | I2��Fe3+��S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������̼ԭ�ӹ�ƽ�� | B�� | �����һ�ȴ��ﹲ��4�� | ||
| C�� | �������4��̼ԭ����һֱ���� | D�� | CnH2n+2�����к���3n+1�����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 | B�� | 3 | C�� | 4 | D�� | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �Ȼ�������Һ��ͨ������������Fe2++Cl2��Fe3++2Cl? | |
| B�� | ��ƫ��������Һ��ͨ�������CO2��AlO2-+2H2O+CO2��Al��OH��3��+HCO3- | |
| C�� | �Ȼ�����Һ�м��������ˮ��Al3++4NH3•H2O��AlO2-+4NH4++2H2O | |
| D�� | �����������м���ϡ���2Al��OH��3+6H+��2Al3++6H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� | 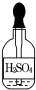 | C�� |  | D�� | 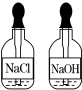 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
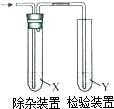 �����з����Ʊ���ϩ��������ͼװ�ü�����ϩ������Ҫ���ӵ��ǣ�������
�����з����Ʊ���ϩ��������ͼװ�ü�����ϩ������Ҫ���ӵ��ǣ�������| ��ϩ���Ʊ� | �Լ�X | �Լ�Y | |
| A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
| B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
| C | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | KMnO4������Һ |
| D | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | Br2��ˮ��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com