
X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�3��Ԫ�ص�ԭ�Ӻ��������֮����Ca2���ĺ����������ȣ�X��Z�ֱ�õ�1�����Ӻ���γ�ϡ������ԭ�ӵ��ȶ����Ӳ�ṹ������˵����ȷ����(����)
A��ԭ�Ӱ뾶��Z��Y��X
B��Z��X�γɻ�����ķе����Z��ͬ��Ԫ����X�γɻ�����ķе�
C��Na2Y2��ˮ����������ԭ��Ӧʱ��Na2Y2ֻ��������
D��CaX2��CaY2��CaZ2��3�ֻ������У��������������Ӹ����Ⱦ�Ϊ1��2
��
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��Ȼ�ѧ����ʽ����д����Ӧ�ı�������ȷ����(����)
A���ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������17.6 g������ʱ���ų�19.12 kJ��������Fe(s)��S(s)===FeS(s)����H����95.6 kJ��mol��1
B��ϡ������0.1 mol��L��1 NaOH��Һ��Ӧ��H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ��mol��1
C����֪1 mol������ȫȼ������Һ̬ˮ���ų�������Ϊ285.5 kJ����ˮ�ֽ���Ȼ�ѧ����ʽΪ2H2O(l)===2H2(g)��O2(g)����H����285.5 kJ��mol��1
D����֪2C(s)��O2(g)===2CO(g)����H����221 kJ��mol��1�����֪C��ȼ���Ȧ�H����110.5 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��W��X��Y��Z��ԭ��������������W��Xԭ�ӵ�����������֮��Ϊ4��3��Zԭ�ӱ�Xԭ�ӵĺ����������4������˵����ȷ����(����)
A��W��Y��Z�ĵ縺�Դ�С˳��һ����Z>Y>W
B��W��X��Y��Z��ԭ�Ӱ뾶��С˳�������W>X>Y>Z
C��Y��Z�γɵķ��ӿռ乹�Ϳ�������������
D��WY2�����к��зǼ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
��ش��������⣺
(1)A��Ԫ�����ڱ��е�λ���ǡ���3���ڢ�A�塡��A��E�γɵĻ�����ĵ���ʽ�ǡ�Mg2��[

 ]2��������������21������ʦ��
]2��������������21������ʦ��
(2)C��ij���Ȼ����Ũ��Һ���Ը�ʴӡˢ��·���ϵĽ���ͭ���˷�Ӧ�����ӷ���ʽ��________________________________________________________________________
________________________________________________________________________��
(3)B�ĵ�����D���⻯����һ�������·�Ӧ����BD����һ����Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)F��������ˮ��Һ�����ԣ�ԭ���ǡ�Al3����3H2O����Al(OH)3��3H����(�����ӷ���ʽ��ʾ)��F�ĵ�����C��D�γɵ���Է�������Ϊ160�Ļ�������һ�������·�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������йػ�ѧ����ʹ����ȷ����(����)
A����ԭ�ӵ�ԭ�ӽṹʾ��ͼ��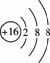
B��NH4Cl�ĵ���ʽ��[H

 H]��Cl��
H]��Cl��
C��ԭ�Ӻ�����10�����ӵ���ԭ�ӣ�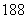 O
O
D�����ȼױ��Ľṹ��ʽ��ClCH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���±��Ƕ������в���Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
| Ԫ�ش��� | U | V | W | X | Y | Z |
| ԭ�Ӱ뾶/nm | 0.037 | 0.157 | 0.066 | 0.070 | 0.077 | 0.143 |
| ��Ҫ���ϼ� | ��1 | ��1 | ��2 | ��3 ��5 | ��2 ��4 | ��3 |
��ش�
(1)X��Ԫ�����ڱ��е�λ���ǡ��ڶ����ڢ�A�塡��
(2)V��W��Z����Ԫ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ�����ߵ����Ӱ뾶�ɴ�С˳����O2��>Na��>Al3����(�����ӷ��ű�ʾ)�������ӷ���ʽ��ʾ��Z���ӿ�����ˮ����ԭ��________________________________________________________________________
________________________________________________________________________��
(3)ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ����Na��[
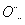
 H]������2��1��c��n��j��y
H]������2��1��c��n��j��y
(4)1 g YU4������ȫȼ������Һ̬ˮʱ���ų�a kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(5)����Z��V2W2�Ĺ���������Ʒ������ϡ�������������ȫ�ܽ⣬���õĻ��Һ��c(Z3��)��c(H��)��c(Cl��)��1��2��8����ԭ���������У�ZԪ����WԪ�ص�������Ϊ��9��16��(���������)������Դ��21�����͡�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������
A�����ǻ�������ķе�����ǻ�������ߣ����мȴ��ڷ��»������ִ������
B���������Ƿ����ò���ά�ѻ��γɵģ����������������ò���ά�ѻ��γɵ�
C�����й��ۼ����з����ԣ��γ�������������ԭ�ӿ��Բ���ֱ����
D����������ĵ��硢�����Զ������ɵ����йأ����Ӿ�����һ�������¿��Ե���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
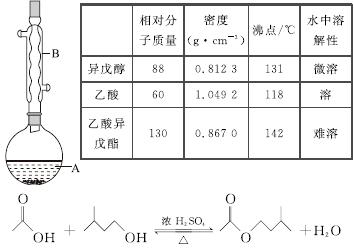
ʵ�鲽�裺
��A�м���4.4 g���촼��6.0 g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50 min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g��
�ش��������⣺
(1)����B��������________________��
(2)��ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����____________________________________���ڶ���ˮϴ����ҪĿ����________________��
(3)��ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��________(����)��
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
(4)��ʵ���м�����������Ŀ����___________________________________________��
(5)ʵ���м���������ˮMgSO4��Ŀ����________��
(6)����������У�����ѡ��װ����ȷ����________(����)��

������a��������������������������b.
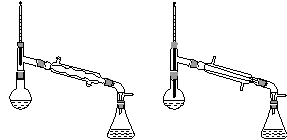
������c��������������������������d.
(7)��ʵ��IJ�����________(����)��
a��30% ��b��40% c��60% d��90%
(8)�ڽ����������ʱ������130 ��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ__________(��ߡ��͡�)����ԭ����______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com