
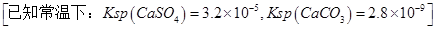 ��
��

 ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
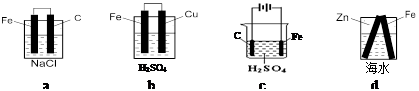
| A��a>b>c>d | B��b>a>d>c | C��d>c>b>a | D��b>d>a>c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
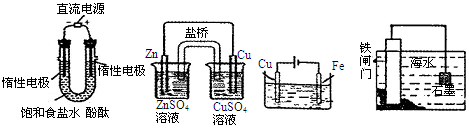
| A��װ�âٹ���ʱ��Һ��OH���������ƶ����������������Һ�ȱ��ɫ |
| B��װ�â���Zn��������Cu������ |
| C������װ�âۿ�ʵ�ֶ�����Ʒ�����ͭ |
| D������װ�âܿ�ʵ�ֶ���բ�ű��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������ڿ�������绯ѧ��ʴ�������䰵 |
| B���ɽ��������ֹ������ֱ����Դ�����������Ա��������ܸ�ʴ |
| C��������ˮ�²��ֱ��ڿ�����ˮ���紦������ʴ |
| D������п����Ʒ�ĶƲ�����Ʋ����ܶ�����Ʒ�𱣻����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

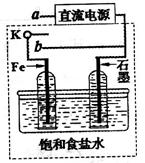
| A�������缫ֱ�����ӣ�a��ʳ��ˮ���������ⸯʴ |
| B�������缫ֱ�����ӣ�����Ӧһ���ǣ�Fe-2e��Fe2�� |
| C�������ӵ�Դ������ʯī�ӵ�Դ������a��ʳ��ˮ�����缫�����������ݱ��Ҳ�缫�ϵĶ� |
| D�������ӵ�Դ������ʯī�ӵ�Դ������a���Ȼ�ͭ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
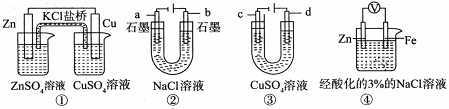
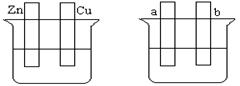
| A��װ�â��У������е� K+����CuSO4��Һ |
| B��װ�â��ڵ������У�a���ϵõ�22.4 L���壨��״��������������Ҫת��NA�����ӣ�NA��ʾ�����ӵ������� |
| C��������װ�â���ͭ�϶�����c��Ϊ�� |
| D��װ�â���һ��ʱ������Fe��OH��2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��Cu | B��CuO | C��Cu(OH)2 | D��Cu2(0H)2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com