
ʵ������NaOH��������250mL 1.25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������250mL 1.25mol/L��NaOH��Һ, Ӧ��ȡNaOH������ g��
��2������ƿ�ϱ��� ����ʹ��ǰ��һ�������� ��
��3����Һע������ƿǰ����ȴ�����£�������Ϊ_____________________________��
��4������ʱ�IJ��� ��
��5���������Ƶ���ҺŨ��ƫ�͵��� ��
| A������NaOHʱ��������������� |
| B��������ƿ��ת����Һʱ������Һ����������ƿ���� |
| C������ʱ���ӿ̶��� |
| D������ǰ������ƿ������������ˮ |
24.��1��12.5 ��2���¶ȣ��ݻ����̶��ߣ�����Ƿ�©ˮ����3��NaOH����ˮʱ���� ��
��4��������ƿ��ע������ˮ����̶���1-2cm�������ý�ͷ�ι���εμ�����Һ����͵���̶��߸պ����У���5�� A ��B ��E��
���������������1�����������Ƶ�����Ϊm=0.25L��1.25mol?L-1��40g/mol=12.5g��
��2������ƿ�Ͽ����¶ȡ��̶��ߺ����Ϊ�����ߵ�ҡ�ȣ���������ƿ��ʹ��ǰ�������Ƿ�©ˮ����Ϊ���������ƿ�Ƿ�©ˮ��
��3������ƿ��Ҫ��������ʹ�ã�NaOH����ˮʱ����,������Һע������ƿǰ��ȴ�����£�
��4������ʱ�IJ���Ϊ������ƿ��ע������ˮ����̶���1-2cm�������ý�ͷ�ι���εμ�����Һ����͵���̶��߸պ����У�
��5��A.����NaOHʱ��������������̣�����ҩƷ���٣�������ҺŨ��ƫ�ͣ�B.������ƿ��ת����Һʱ������Һ����������ƿ���棬�������ʼ��٣�������ҺŨ��ƫ�ͣ�C.����ʱ���ӿ̶��ߣ���Һ������٣�������ҺŨ��ƫ�ߣ�D.����ǰ������ƿ������������ˮ����Ӱ�죻E.���ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ�������Һ�������������ҺŨ��ƫ�ͣ�ѡA ��B ��E.
���㣺����һ�����ʵ���Ũ����Һ�����ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ʹ�õĻ��˵�̺�����ϰ塢����Ȼ�����Ʒ�����ͷų�ij����Ⱦ���������壬��������
| A����ȩ | B���������� | C������ | D���Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ӳˮ����ˮ�ı���������
| A��Ӳˮ���ǡ���ˮ���� |
| B��Ӳˮ�������ʡ���ˮ�������� |
| C��Ӳˮ���н϶�����Ը�þ�������ˮ���������������Ը�þ������ |
| D��Ӳˮ�Dz�����ˮ����ˮ�Ǵ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣�������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��ŨH2SO4������500 mL0.25 mol/L��ϡH2SO4��
�ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ������ƿ ��������ƽ ��ҩ�ס�
��ش��������⣺
��1�����������У�������ϡH2SO4ʱ�ò������� (�����)��
��2�������㣬��ŨH2SO4�����Ϊ �����Т�10 mL ��50 mL ��100 mL���ֹ�����Ͳ��Ӧѡ�õ���Ͳ�� (�����)��
��3����ŨH2SO4����������ˮϡ�ͣ���ȴƬ�̣����ȫ��ת�Ƶ� mL������ƿ�У�ת��ʱӦ�ò����� ��ת����ϣ�����������ˮϴ�� 2��3�Σ�����ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ��������ƿ��ʹ��Һ��Ͼ��ȡ�Ȼ���ذ�����ˮֱ��ע������ƿֱ��Һ��ӽ��̶ȴ������� ������ˮ��ƿ���̶ȵĵط���ʹ��Һ�� ����ҡ�Ⱥ�װƿ����ǩ��
��4�������ƹ����У�����������ȷ�����в����У����������ƫ�ߵ��� (�����)��
��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ��
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ��
�۽�ŨH2SO4ֱ�ӵ����ձ��������ձ���ע������ˮϡ��ŨH2SO4
�ܶ���ʱ��������ˮ�������ߣ����ý�ͷ�ι�����
��ת��ǰ������ƿ�к�����������ˮ
����ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ������
�߶���ʱ�����ӱ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������NaOH��������250mL 1��25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������ƿ����������������е� ����3�֣�
���¶� ��Ũ�� ������ ��ѹǿ �ݿ̶���
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ�� ����3�֣�
����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
�۽�����ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
�ܽ�����ƿ�ǽ����ߵ�ҡ��
�ݸ��ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
����ʱ�������Ҫʵ�������� ��5�֣�
��4����6�֣����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
����ֽ����NaOH���塣 ____________;
��������ƿ��ˮ���ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ�����ȡ�κδ�ʩ��___;
��������NaOH��Һ�������ձ��ڡ�____________;
��������ƿ��ˮʱ�۾�һֱ����Һ�档____________;
������ǰ������ƿ������������ˮ��____________;
����NaOHʱ��������������̡�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����13�֣�ʵ������Ҫ90mL 2.0 mol��L��1��Na2CO3��Һ��������ˮ̼���Ʒ�δ���ƣ���ش��������⣺
��1������ͨ�����㣨Ҫ�м�����̣�����ȷ����ȡ g��ˮ̼���ơ�
��2�����������У������õ�����
A��50mL����ƿ�� B��100mL����ƿ�� C��������
D��100mL��Ͳ�� E��������ƽ�� F��ҩ��
��3����Ҫʵʩ���ƣ������������⣬��ȱ�������� ��
��4������ƿ��ʹ��ǰ������еIJ����� ��
��5�����ƹ��̼���Ϊ���¸���������ȷ�IJ���˳��Ϊ (����������)��
A����ȴ�����£� B��ϴ�Ӳ���Һ�� C����ȡ�� D���ܽ⣻
E��ҡ��װƿ�� F�����ݣ� G����Һ
��6�������ƹ����У����������Ũ���к�Ӱ��?
������ƿ������ˮϴ����û�ȵ������������Һ���ݣ���������Һ��Ũ�� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����
�� ת����Һʱ����С������Һ����ƿ�⣬��������Һ��Ũ��
�۶���ʱ�����ӿ̶��ߣ���������Һ��Ũ��
�����ڵμ�����ˮʱ�����������˿̶��ߣ���������Һ��Ũ�� ����ʱӦ��δ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣�ʵ����Ҫ����480 mL 0.2 mol/L NaOH��Һ����ش��������⣺
��1�����ƹ���������Ҫʹ�õĻ�ѧ������__________________����ѡ�����ĸ����
A���ձ� B��500 mL����ƿ C��©�� D����ͷ�ι� E��������
��2����������ƽ��ȡ�������ƣ�������Ϊ_______________g��
��3��ȡ����������ĸ�NaOH��Һʱ�������������в�����ȡ����Ķ��ٶ��仯����_______��
A����Һ��NaOH�����ʵ��� B����Һ��Ũ��
C����Һ��OH������Ŀ D����Һ���ܶ�
��4��������������Һ�Ĺ����У���������� NaOH��Һ���ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�������Ӱ�족��
��δ����ȴ���Ƚ���Һע������ƿ�У�______________
������ƿ������ϴ�Ӻ������������ˮ��_____________
�۶���ʱijͬѧ�۲�Һ��������ͼ��ʾ���������Ƶ���Һ��Ũ��____________��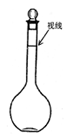
��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���___________������ƫ�͡�������Ӱ�족��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��8�֣�����500mL 0.5mol��L-1��NaOH��Һ���Իش��������⣺
��1�����㣺��ҪNaOH���������Ϊ ��
��2��ijѧ����������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽� (����ڡ����ڡ�)�ұߵ����̡�
��3�����Ʒ������������������裺
����ʢ��NaOH���ձ��м���200mL����ˮʹ���ܽ⣬����ȴ�����¡�
�ڼ���������ƿ�м�����ˮ��Һ��ӽ��̶���1cm��2cm��
�۽�NaOH��Һ�ز�����ע��500mL����ƿ�С�
�����ձ��м�������������ˮ��С��ϴ��2��3�κ���������ƿ��
�ݸ��ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
�Խ�����˳������ȷ���� ��
A���٢ۢڢܢ� B���٢ܢۢڢ� C���٢ۢܢڢ� D���٢ܢڢۢ�
��4��ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48mol��L-1�������ܵ�ԭ����
A��ʹ����ֽ�����������ƹ���
B������ƿ��ԭ��������������ˮ
C��ת����Һ����ձ�δ�����ϴ��
D����ͷ�ιܼ�ˮ����ʱ���ӿ̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������Ͷ�뵽�����У�������ų�����

























| A��Na | B��Na2O | C��Na2O2 | D��Na2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com