
(11��) ��900oC�Ŀ����кϳɳ�һ�ֺ��硢�ƺ��� (Ħ����2 : 2 : 1) �ĸ�������������̿����� +2��+3��+4 ����ϼ�̬���ڡ�Ϊȷ���ø���������Ļ�ѧʽ���������·�����
6-1 ȷ��ȡ25.00 mL 0.05301 mol L1�IJ�����ˮ��Һ��������ƿ�У�����25 mL����ˮ��5 mL 6 mol L1��HNO3��Һ������60~70oC����KMnO4��Һ�ζ�������27.75 mL��д���ζ����̷����ķ�Ӧ�ķ���ʽ������KMnO4��Һ��Ũ�ȡ�
6-2 ȷ��ȡ0.4460 g������������Ʒ��������ƿ�У���25.00 mL������������Һ��30 mL 6 mol L1 ��HNO3��Һ����60~70oC�³��ҡ����Լ��Сʱ��õ���ɫ����Һ��������KMnO4��Һ�ζ�������10.02 mL������ʵ�������㸴�����������̵ļ�̬�������ø���������Ļ�ѧʽ��д����Ʒ�ܽ���̵ķ�Ӧ����ʽ����֪La��ԭ����Ϊ138.9��
6-1 2MnO4- + 5C2O42- + 16H+ = 2Mn2+ + 10CO2 + 8H2O (1��)
KMnO4��ҺŨ�ȣ�(2/5) ´ 0.05301 ´ 25.00 / 27.75 = 0.01910 ��mol L-1�� (1��)
��Ӧ����ʽ��Ӧ��C2O42-дH2C2O4��ֻҪ��ƽ��Ҳ��1�֡���ͬ��
6-2���ݣ��������н�������Ħ����Ϊ La : Ca : Mn = 2 : 2 : 1����Ƶ�����̬�ֱ�Ϊ+3��+2���̵�����̬Ϊ +2 ~ +4�������ж�
����������Ļ�ѧʽΪLa2Ca2MnO6+x, ���� x = 0~1�� (1��)
������
����C2O42������25.00 mL ´ 0.05301 mol L1 = 1.3253 mmol (0.5��)
��Ʒ�ܽ�ζ����ĸ�����أ�10.02 mL ´ 0.01910 mol L1 = 0.1914 mmol (0.5��)
��Ʒ�ܽ��ʣ��C2O42��: 0.1914 mmol ´ 5/2 = 0.4785 mmol (0.5��)
��Ʒ�ܽ���������ĵ�C2O42��: 1.3253 mmol 0.4785 mmol = 0.8468 mmol (0.5��)
�������Ϲ��̺ϲ������ۺ����������ȷ��Ҳ��2�֡�
�����������У�C2O42��ΪCO2�������ӣ�
2 ´ 0.8468 mmol = 1.694 mmol (0.5��)
���������x�ķ�����
��1�����̷���
����������(La2Ca2MnO6+x)��Ʒ�����ʵ���Ϊ��
0.4460 g / [(508.9 + 16.0 x) g mol-1] (0.5��)
La2Ca2MnO6+x�У��̵ļ�̬Ϊ: [2 ´ (6+x) -2 ´3 -2´2] = (2+2x) (1��)
�����������̵ļ�̬�仯Ϊ�� (2+2 x - 2) = 2 x (0.5��)
�̵õ�������C2O42����������ȣ�
2 x ´ 0.4460 g / [(508.9 + 16.0 x) g mol-1] = 2 ´ 0.8468 ´ 10-3 mol (1��)
x = 1.012 » 1 (0.5��)
��������ϲ���ϲ����Ƶ������������ȷ��Ҳ��3.5�֣���������ϲ���ϲ����Ƶ��������������2�֣��Ƶ����������Ǻϣ�Ҳ���÷֡�
��2�����Է�
��Ϊ���������������൱����C2O42-���ɼ��̵ļ�̬�϶�������+2�ۡ������̵ļ�̬Ϊ+3�ۣ���Ӧ������Ļ�ѧʽΪLa2Ca2MnO6.5, �˻�ѧʽʽ��Ϊ516.9 g mol-1, ��ȡ��Ʒ�����ʵ���Ϊ��
0.4460 g / (516.9 g mol-1) = 8.628 ´10-4 mol (0.5��)
�������������̵ļ�̬�仯Ϊ
1.689 ´ 10-3 mol / (8. 628 ´10-4 mol) = 1.96 (0. 5��)
���ڸ����������еļ�̬Ϊ�� 2 + 1.96 = 3.96 (0. 5��)
3.96��3���ܴ�+3�ۼ��費������ (0.5��)
�������ʾMn���ӽ���+4�ۡ� (0.5��)
����MnΪ+4��, ��Ӧ������Ļ�ѧʽΪLa2Ca2MnO7, �˻�ѧʽʽ��Ϊ524.9 g mol-1��
���ڸ����������еļ�̬Ϊ��
2 + 2 ´ 0.8468 ´ 10-3 / (0.4460 / 524.9) = 3.99 » 4 (0.5��)
���������Ǻϣ��ɼ��ڸ����������У�MnΪ+4�ۡ� (0.5��)
��������ϲ���ϲ����Ƶ������������ȷ��Ҳ��3.5�֣���������ϲ���ϲ����Ƶ��������������2�֣��Ƶ����������Ǻϣ�Ҳ���÷֡�
�ø���������Ļ�ѧʽΪLa2Ca2MnO7 (1��)
�������̵ķ�Ӧ����ʽΪ��
La2Ca2MnO7 + C2O42- + 14H+ = 2La3+ + 2Ca2+ + Mn2+ + 2CO2 + 7H2O (1��)
δ������ó�La2Ca2MnO7������ʽ��ȷ��ֻ�÷���ʽ��1�֡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������һ����顡���л�ѧ������2��(���հ�α걾) ���հ�α걾 ���ͣ�022
2001��9��11�գ��ֲ����Ӽ�ʻ����757�ɻ���900 km/h���ٶ�ײ��ŦԼ��ó���ã���֪����757�ɻ���80 m��������110 t������Я������ú��30 t��ú�͵�ȼ��ֵΪ4.62��104kJ/kg ������ú����ʯ�ͷ����Ʒ֮һ������Ҫ�ɷ��Ƿ����к�̼ԭ�Ӹ���10��15���������ɻ�ײ������ʱ��ú����ȼ�ձ�ը�������ĸ��������£���һ����Ѹ�ٷ����ѻ�������̼ԭ�Ӹ��ٵ������Ӿ��˱�ը�̶ȣ�
(1)����ú�ͳɷ�֮һA�ķ����к���̼ԭ��nA���������ѻ�ΪB��C��������������зֱ�̼ԭ��nB��nC������BΪ�������������������ȷ����________(����ţ���ѡ���ѡ���۷�)��
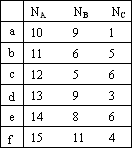
(2)��д������b�����õ���ϩ���У���������4��̼ԭ�ӵ�ͬ���칹��Ľṹ��ʽ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
(1)����ú�ͳɷ�֮һA�ķ����к���̼ԭ��n(A)���������ѻ�ΪB��C��������������зֱ�̼ԭ��n(B)��n(C)������BΪ�������������������ȷ����_____________________(����ţ���ѡ���ѡ���۷�)��
| n(A) n(B) n(C) |
a | 10 9 1 |
b | 11 6 5 |
c | 12 5 6 |
d | 13 9 3 |
e | 14 8 6 |
f | 15 11 4 |
(2)��д������b�����õ���ϩ���У���������4��̼ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com