

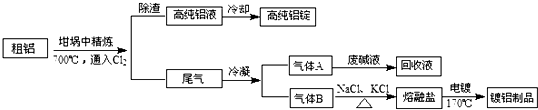
·ÖĪö £Ø1£©µē½ā¾«Į¶“ÖĶŹ±£¬“ÖĶÖŠµÄCuŗĶÉŁĮæZnŌŚŃō¼«·¢ÉśŃõ»Æ·“Ó¦£¬Zn±ČCu»īĘĆ£¬ĻČŹ§µē×Ó£¬ĖłŅŌŃō¼«µē¼«·“Ó¦Ź½ĪŖZn-2e-=Zn2+£»Cu-2e-=Cu2+
£Ø2£©øł¾ŻTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬Ī¢ČÜÓŚĖ®£¬¼Ó¼īČܹżĀĖ³żČ„ŌÓÖŹ£¬µĆµ½Na2TeO3ČÜŅŗ£¬ŌŁ¼ÓĮņĖį³Į½µ¾¹żĀĖµĆµ½TeO2³Įµķ£¬ŌŁÓĆŃĪĖįČܽāÉś³ÉĖÄĀČ»ÆķŚ£¬ŌŁÓƶžŃõ»ÆĮņ»¹ŌÖĘ³ÉķŚµ„ÖŹ£¬ŅŌ“Ė·ÖĪö½ā“š£»
¢ŁTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬ÓėĒāŃõ»ÆÄĘ·¢ÉśĄąĖĘŃõ»ÆĀĮÓėĒāŃõ»ÆÄʵķ“Ó¦£»
¢ŚŅņĪŖTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬H2SO4¹żĮæ»įµ¼ÖĀTeO2¼ĢŠųÓėH2SO4·“Ó¦µ¼ÖĀĖšŹ§£¬ÓĆĻõĖįļ§Ģę“śĮņĖį£¬·¢Éś·“Ó¦»įÉś³ÉĻõĖįÄĘŗĶTeO2£»
¢ŪSO2»¹ŌTeCl4ĪŖTe£¬±¾Éķ±»Ńõ»ÆĪŖĮņĖį£¬øł¾ŻµĆŹ§µē×ÓŹŲŗćŹéŠ“£¬øł¾ŻķŚŌŖĖŲŹŲŗćŗĶµē×ӵƏ§ŹŲŗć¼ĘĖ涞Ńõ»ÆĮņµÄĢå»ż£»
½ā“š ½ā£ŗ£Ø1£©µē½ā¾«Į¶“ÖĶŹ±£¬“ÖĶÖŠµÄCuŗĶÉŁĮæZnŌŚŃō¼«·¢ÉśŃõ»Æ·“Ó¦£¬Zn±ČCu»īĘĆ£¬ĻČŹ§µē×Ó£¬ĖłŅŌŃō¼«µē¼«·“Ó¦Ź½ĪŖZn-2e-=Zn2+”¢Cu-2e-=Cu2+£¬
¹Ź“š°øĪŖ£ŗZn-2e-=Zn2+”¢Cu-2e-=Cu2+£»
£Ø2£©øł¾ŻTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬Ī¢ČÜÓŚĖ®£¬¼Ó¼īČܹżĀĖ³żČ„ŌÓÖŹ£¬µĆµ½Na2TeO3ČÜŅŗ£¬ŌŁ¼ÓĮņĖį³Į½µ¾¹żĀĖµĆµ½TeO2³Įµķ£¬ŌŁÓĆŃĪĖįČܽāÉś³ÉĖÄĀČ»ÆķŚ£¬ŌŁÓƶžŃõ»ÆĮņ»¹ŌÖĘ³ÉķŚµ„ÖŹ£»
¢ŁTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬ÓėĒāŃõ»ÆÄĘ·¢ÉśĄąĖĘŃõ»ÆĀĮÓėĒāŃõ»ÆÄʵķ“Ó¦£¬»Æѧ·½³ĢŹ½ĪŖTeO2+2NaOH=Na2TeO3+H2O£¬
¹Ź“š°øĪŖ£ŗTeO2+2NaOH=Na2TeO3+H2O£»
¢ŚŅņĪŖTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬H2SO4¹żĮæ»įµ¼ÖĀTeO2¼ĢŠųÓėH2SO4·“Ó¦µ¼ÖĀĖšŹ§£»·ĄÖ¹¾Ö²æĖį¶Č¹ż“óµÄ²Ł×÷·½·ØŹĒ£ŗ»ŗĀż¼ÓČėH2SO4£¬²¢²»¶Ļ½Į°č£¬ÓĆĻõĖįļ§Ģę“śĮņĖį£¬·¢Éś·“Ó¦»įÉś³ÉĻõĖįÄĘŗĶTeO2£¬·“Ó¦·½³ĢŹ½ĪŖNa2TeO3+2NH4NO3ØT2NaNO3+TeO2”ż+2NH3+H2O£¬
¹Ź“š°øĪŖ£ŗTeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬H2SO4¹żĮæ»įµ¼ÖĀTeO2¼ĢŠųÓėH2SO4·“Ó¦µ¼ÖĀĖšŹ§£»»ŗĀż¼ÓČėH2SO4£¬²¢²»¶Ļ½Į°č£»Na2TeO3+2NH4NO3ØT2NaNO3+TeO2”ż+2NH3+H2O£»
¢ŪSO2»¹ŌTeCl4ĪŖTe£¬±¾Éķ±»Ńõ»ÆĪŖĮņĖį£¬»Æѧ·½³ĢŹ½ĪŖTeCl4+2SO2+4H2O=Te+4HCl+2H2SO4£¬Ńō¼«ÄąÖŠŗ¬TeO2µÄÖŹĮæ·ÖŹżĪŖ8%£¬ĢįČ”¹ż³ĢÖŠķŚŌŖĖŲµÄ»ŲŹÕĀŹĪŖ90%£¬Ōņ“¦Ąķ1tŃō¼«ÄąæɵĆTeCl4µÄÖŹĮæĪŖ£Ø1”Į90%”Į8%£©”Į$\frac{190}{80}$t=0.171t£¬ĘäĪļÖŹµÄĮæĪŖ900mol£¬øł¾Ż·½³ĢŹ½ĄūÓƵē×ӵƏ§ŹŲŗćæÉÖŖ¶žŃõ»ÆĮņµÄĪļÖŹµÄĮæĪŖ1800mol£¬ĘäĢå»żĪŖ40320L£¬
¹Ź“š°øĪŖ£ŗTeCl4+2SO2+4H2O=Te+4HCl+2H2SO4£»40320£®
µćĘĄ ±¾Ģāæ¼²é“ÖĶ¾«Į¶µÄµē½ā·“Ó¦Ź½µÄŹéŠ“£¬¶Ōøų¶ØĢõ¼žµÄ»Æѧ·½³ĢŹ½”¢Ąė×Ó·½³ĢŹ½µÄÅŠ¶Ļ¼°ŹéŠ“£¬ŹµŃé²Ł×÷µÄÅŠ¶ĻŹĒ½ā±¾Ģā¹Ų¼ü£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬ĖüæÉŅŌĶعż²»Ķ¬µÄ·“Ó¦µĆµ½ĻĀĮŠĪļÖŹ£ŗ
£¬ĖüæÉŅŌĶعż²»Ķ¬µÄ·“Ó¦µĆµ½ĻĀĮŠĪļÖŹ£ŗ C£®
C£® D£®
D£®
 +H2O£®
+H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ź±¼ä/s | 0 | 20 | 40 | 60 | 80 | 100 |
| c£ØNO2£©/mol•L-1 | 0.00 | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
| A£® | ÉżøßĪĀ¶ČøĆ·“Ó¦µÄĘ½ŗā³£ŹżK¼õŠ” | |
| B£® | 20”«40 s ÄŚ£¬v£ØN2O4£©=0.004 mol•L-1•s1 | |
| C£® | ·“Ó¦“ļĘ½ŗāŹ±£¬ĪüŹÕµÄČČĮæĪŖ0.30 Q kJ | |
| D£® | 100 s Ź±ŌŁĶØČė0.40 mol N2O4£¬“ļŠĀĘ½ŗāŹ±N2O4µÄ×Ŗ»ÆĀŹŌö“ó |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com