
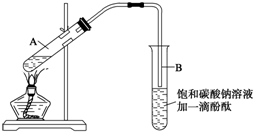
·ÖĪö £Ø1£©×é×°ŅĒĘ÷µÄŅ»°ćĖ³ŠņŹĒ²ÉÓĆĻČĻĀŗóÉĻ£¬ĻČ×óŗóÓŅµÄŌŌņ£¬ĄūÓƱ¾¹ęŌņ¼“æɽā“š£»
£Ø2£©¼ÓČėĀČ»Æ±µČÜŅŗ¼ģŃéĮņĖįøłĄė×ÓŹĒ·ń³ż¾”£»
£Ø3£©ŌŚ“ÖŃĪĢį“æµÄ¹ż³ĢÖŠŠčŅŖµĪ¼Ó±„ŗĶNa2CO3ČÜŅŗ³żČ„øĘĄė×ÓŅŌ¼°¹żĮæµÄ±µĄė×Ó£»
£Ø4£©¹żĀĖ³żČ„Ē°Ćę¼Ó³Įµķ¼ĮÉś³ÉµÄ³Įµķ£»
£Ø5£©ČܽāŹ±ĪŖ¼ÓæģČܽā”¢¹żĀĖŹ±ĪŖ·ĄÖ¹ŅŗĢåĶāČ÷”¢³åĘĘĀĖÖ½¶ųŅżĮ÷”¢Õō·¢Ź±ĪŖ·ĄÖ¹ŅŗĢ彦³ö¶¼ŅŖŹ¹ÓĆ²£Į§°ō£®
½ā“š ½ā£ŗ£Ø1£©×é×°ŅĒĘ÷Ź±ŅŖ“ÓĻĀĻņÉĻ×é×°£¬¾Ę¾«µĘŌŚĢśČ¦ŗĶÕō·¢ĆóµÄĻĀ·½£¬ĖłŅŌŅŖĻČ·Å¾Ę¾«µĘ£»Č»ŗóŌŁ¹Ģ¶ØĢśČ¦£¬·ÅÖĆÕō·¢Ćó£»Č»ŗóŌŁµćČ¼¾Ę¾«µĘ¼ÓČČ£¬²¢½Į°č£¬µ±ÓŠ½Ļ¶ą¾§ĢåĪö³öŹ±£¬Ķ£Ö¹¼ÓČČ£¬½čÓąČČÕōøÉ£®ÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ¢Ł¢Ś¢Ū¢Ü¢Ż£»
¹Ź“š°øĪŖ£ŗ¢Ł¢Ś¢Ū¢Ü¢Ż£»
£Ø2£©¼ÓČė¹żĮæĀČ»Æ±µČÜŅŗ³żČ„ĮņĖįøłĄė×Ó£¬¼ģŃéĮņĖįøłĄė×ÓŅŃ³ż¾”£¬æɾ²Ö¹Ę¬æĢŌŚÉĻ²ćĒåŅŗ“¦£¬µĪ¼ÓŅ»µĪĀČ»Æ±µČÜŅŗ£¬²»³öĻÖ»ė×Ē¾ĶĖµĆ÷ĮņĖįøłĄė×ÓŅŃ¾³ż¾”£¬Čē¹ū»¹ÓŠ£¬æÉŅŌŌŚČÜŅŗÖŠ¼ĢŠų¼ÓČė¹żĮæµÄĀČ»Æ±µ£¬
¹Ź“š°øĪŖ£ŗ¾²ÖĆʬæĢŗó£¬Č”ÉĻ²ćĒåŅŗÓŚŹŌ¹ÜÖŠ£¬¼ĢŠųµĪ¼ÓĀČ»Æ±µ£¬Čō³öĻÖ»ė×Ē»ņ°×É«³Įµķ£¬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠĮņĖįøł£¬·ńŌņ²»ŗ¬ĮņĖįøł£»ŌŚČÜŅŗÖŠ¼ĢŠų¼ÓČė¹żĮæµÄĀČ»Æ±µ£»
£Ø3£©ĒāŃõ»ÆÄĘÓėĆ¾Ąė×ÓÉś³ÉĒāŃõ»ÆĆ¾³Įµķ”¢ÓėøĘĄė×ÓÉś³ÉĢ¼ĖįøĘ³Įµķ”¢Óė±µĄė×ÓÉś³ÉĢ¼Ėį±µ³Įµķ£¬ĖłŅŌµĪ¼Ó±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬æÉŅŌ³żČ„øĘĄė×Ó”¢Ć¾Ąė×ÓŗĶ¹żĮæµÄ±µĄė×Ó£¬
¹Ź“š°øĪŖ£ŗ³żČ„Ca2+”¢Mg2+¼°¼ÓČė¹żĮæµÄBa2+£»
£Ø4£©¹żĀĖ³żČ„Ē°Ćę¼Ó³Įµķ¼ĮÉś³ÉµÄ³Įµķ£¬ÓŠBaSO4”¢CaCO3”¢Mg£ØOH£©2”¢BaCO3µČŌÓÖŹ£»
¹Ź“š°øĪŖ£ŗBaSO4”¢CaCO3”¢Mg£ØOH£©2”¢BaCO3µČŌÓÖŹ£»
£Ø5£©ŌŚČܽā²Ł×÷ÖŠ£¬²£Į§°ōĘšµ½½Į°čŅŌ¼ÓæģŹ³ŃĪµÄČܽāµÄ×÷ÓĆ£»
¹żĀĖ²Ł×÷ÖŠ£¬²£Į§°ōÓĆŅŌŅżĮ÷¶ų·ĄÖ¹ŅŗĢåĶāČ÷£¬³åĘĘĀĖÖ½£»
Õō·¢²Ł×÷ÖŠ£¬²£Į§°ōĘšµ½½Į°č¶ųŹ¹ŅŗĢåŹÜČČ¾łŌČ£¬·ĄÖ¹Ņņ¾Ö²æ¹żČČŅŗµĪ»ņ¾§Ģå·É½¦£»
¹Ź“š°øĪŖ£ŗ½Į°č£¬¼ÓĖŁČܽā£»ŅżĮ÷£»½Į°č£¬·ĄÖ¹¾Ö²æ¹żČČ£¬ŅŗĢå·É½¦£®
µćĘĄ ±¾Ģāæ¼²éŌŚ“ÖŃĪĢį“æµÄ¹ż³ĢÖŠĖłŃ”ÓĆ³żŌÓŗĶ¾»»ÆµÄ·½·Ø£¬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņā³żČ„ŌÓÖŹ²»ŅŖŅżČėŠĀµÄŌÓÖŹ£¬¶ŌÓŚŹµŃé¹ż³ĢÖŠ¹żĮæµÄŹŌ¼Į¶¼ŅŖ³żČ„£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦¼°»ÆѧŹµŃéÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŌĘÄĻŹ”øßŅ»9ŌĀŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ÖŠ£¬ÕżČ·µÄŹĒ
A£®Ļ”ĮņĖįµĪŌŚŅųʬÉĻ £ŗ2Ag + 2H+£½2Ag+ + H2”ü
£ŗ2Ag + 2H+£½2Ag+ + H2”ü
B£®ÄĘŗĶĄäĖ®·“Ó¦£ŗNa£«2H2O£½Na£«£«2OH?£«H2”ü
C£®³ĪĒåŹÆ»ŅĖ®Óė×ćĮæµÄĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦£ŗCa2+ + OH- + HCO3-£½CaCO3”ż + H2O
D£®Ńõ»ÆĢśÓėĻ”ŃĪĖį »ģŗĻ£ŗFe2O3 + 6H+£½2Fe3+ + 3H2O
»ģŗĻ£ŗFe2O3 + 6H+£½2Fe3+ + 3H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ°²»ÕŹ¦“óø½ÖŠø߶žÉĻ10ŌĀŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠø÷×éČČ»Æѧ·½³ĢŹ½ÖŠ¦¤H1Š”ÓŚ¦¤H2µÄŹĒ
£Ø1£©S£Øs£©£«O2£Øg£©===SO2£Øg£©£¬¦¤H1
S£Øg£©£«O2£Øg£©===SO2£Øg£©£¬¦¤H2
£Ø2£©CH4£Øg£©£«2O2£Øg£©===CO2£Øg£©£«2H2O£Øl£©£¬¦¤H1
CH4£Øg£©£«1.5O2£Øg£©===CO£Øg£©£«2H2O£Øg£©£¬¦¤H2
£Ø3£©4Al£Øs£©£«3O2£Øg£©===2Al2O3£Øs£©£¬¦¤H1
4Fe£Øs£©£«3O2£Øg£©===2Fe2O3£Øs£©£¬¦¤H2
£Ø4£©·ÅČČ·“Ó¦CO£Øg£©+2H2£Øg£© CH3OH£Øg£©¦¤H1
CH3OH£Øg£©¦¤H1
CO£Øg£©+2H2£Øg£© CH3OH£Øl£©¦¤H2
CH3OH£Øl£©¦¤H2
A£®Ö»ÓŠ£Ø2£© B£®Ö»ÓŠ£Ø2 £© £Ø4£©
C£®Ö»ÓŠ£Ø1£© £Ø2 £© £Ø4£© D£®Ö»ÓŠ£Ø2 £© £Ø3£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŅŃÖŖĻĀĮŠŹż¾Ż£ŗ
ŅŃÖŖĻĀĮŠŹż¾Ż£ŗ| ĪļÖŹ | ČŪµć£Ø”ę£© | ·Šµć£Ø”ę£© | ĆÜ¶Č£Øg•cm-3£© |
| ŅŅ“¼ | -117.0 | 78.0 | 0.79 |
| ŅŅĖį | 16.6 | 117.9 | 1.05 |
| ŅŅĖįŅŅõ„ | -83.6 | 77.5 | 0.90 |
| ÅØĮņĖį£Ø98%£© | - | 338.0 | 1.84 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ŅŃÖŖČē±ķŹż¾Ż£ŗ
ŅŃÖŖČē±ķŹż¾Ż£ŗ| Īļ ÖŹ | 2£¬4£¬6 ČŪµć/”ę | ·Šµć/”ę | ĆܶČ/g•cm-3 |
| ŅŅ “¼ | -114 | 78 | 0.789 |
| ŅŅ Ėį | 16.6 | 117.9 | 1.05 |
| ŅŅĖįŅŅõ„ | -83.6 | 77.5 | 0.900 |
| ÅØH2SO4 | 338 | 1.84 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā


| ŹµŃ鱹ŗÅ | ŹŌ¹Ü¼×ÖŠŹŌ¼Į | ŹŌ¹ÜŅŅÖŠŹŌ¼Į | ÓŠ»ś²ćµÄŗń¶Č/cm |
| A | 2 mLŅŅ“¼”¢1 mLŅŅĖį”¢ 1mL18mol•L-1 ÅØĮņĖį | ±„ŗĶNa2CO3ČÜŅŗ | 3.0 |
| B | 2 mLŅŅ“¼”¢1 mLŅŅĖį | 0.1 | |
| C | 2 mLŅŅ“¼”¢1 mLŅŅĖį”¢ 3 mL 2mol•L-1 H2SO4 | 0.6 | |
| D | 2 mLŅŅ“¼”¢1 mLŅŅĖį”¢ŃĪĖį | 0.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
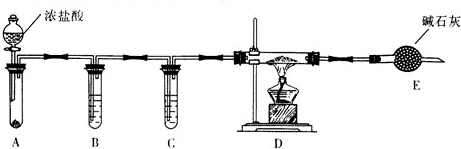
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
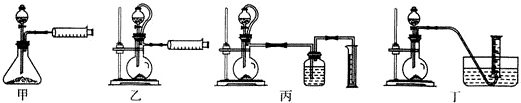
 ”°¾ĘŹĒ³ĀµÄĻć”±£¬ŹĒŅņĪŖ¾ĘŌŚ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£®ŌŚŹµŃéŹŅĪŅĆĒæÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĄ“ÖĘČ”ŅŅĖįŅŅõ„£®»Ų“šĻĀĮŠĪŹĢā£ŗ
”°¾ĘŹĒ³ĀµÄĻć”±£¬ŹĒŅņĪŖ¾ĘŌŚ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£®ŌŚŹµŃéŹŅĪŅĆĒæÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĄ“ÖĘČ”ŅŅĖįŅŅõ„£®»Ų“šĻĀĮŠĪŹĢā£ŗ CH3COOCH2CH3+H2O£®
CH3COOCH2CH3+H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·“Ó¦¢ŁÖŠµēÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ | |
| B£® | ·“Ó¦¢ŚĪŖ·ÅČČ·“Ó¦ | |
| C£® | ·“Ó¦¢ŪŹ¹ÓĆ“ß»Æ¼Į£¬”÷H3¼õŠ” | |
| D£® | ·“Ó¦CH4£Øg£©=C£Øs£©+2H2£Øg£©µÄ”÷H3=74.8kJ•mol-1 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com