
 ��0.1mol��þ�������������100mL 2mol/LH2SO4��Һ�У�Ȼ���ٵμ�1mol/L NaOH��Һ����ش�
��0.1mol��þ�������������100mL 2mol/LH2SO4��Һ�У�Ȼ���ٵμ�1mol/L NaOH��Һ����ش�| 1 |
| 2 |
| 1 |
| 2 |
| 0.46mol |
| 1mol/L |
| 0.4mol |
| 1mol/L |


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1.0 mol�� 10% |
| B��2.0 mol�� 80% |
| C��2.0 mol�� 40% |
| D��2.0 mol�� 20% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ȼ��� | B���Ȼ��� |
| C���Ȼ�þ | D���Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4��C3H4�������Ϊ1��1 |
| B��C2H2��C2H6�������Ϊ3��1 |
| C��C2H4��C2H6�������Ϊ2��1 |
| D��C2H2 ��C2H4�������Ϊ2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������̼ | B��һ����̼ |
| C���������� | D������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D | |
| װ�� | 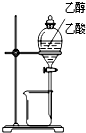 | 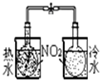 | 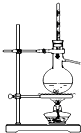 |  |
| ʵ�� | �����Ҵ������� | ֤���¶ȶԻ�ѧƽ���Ӱ�� | ����е����ϴ�Ļ���Һ������ | ��ȡ���ռ����� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ⱥ�۲��Ƿ���������� |
| B������ˮ��μ�ϡ�Ȼ�����Һ�۲��Ƿ��г������� |
| C������ˮ�������Һ�Ƿ��Լ��� |
| D������ˮ��ͨ��CO2�۲��Ƿ���NaHCO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
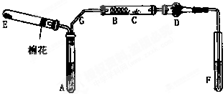 ��ͼ��ij��ѧ��ȤС����Ƶ��Ҵ���������ʵ��װ�ã�ͼ�м�������������̨���Թܼо�δ��������ͼ��A��Ϊ��ˮ�Ҵ����е�78�棩��B��Ϊ�Ƴ�����״��ϸͭ˿����˿��C��Ϊ��ˮCuSO4��ĩ��D��Ϊ��ʯ�ң�F��Ϊ���Ƶļ���Cu��OH��2����Һ��
��ͼ��ij��ѧ��ȤС����Ƶ��Ҵ���������ʵ��װ�ã�ͼ�м�������������̨���Թܼо�δ��������ͼ��A��Ϊ��ˮ�Ҵ����е�78�棩��B��Ϊ�Ƴ�����״��ϸͭ˿����˿��C��Ϊ��ˮCuSO4��ĩ��D��Ϊ��ʯ�ң�F��Ϊ���Ƶļ���Cu��OH��2����Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������1��1 |
| B����1��1 |
| C������1��1 |
| D��С�ڻ����1��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com