
�������(K2FeO4)��һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
(1)�ɷ��Ʊ�������ص���Ҫ��ӦΪ��2FeSO4 �� 6Na2O2===2Na2FeO4 �� 2Na2O �� 2Na2SO4 �� O2��
�ٸ÷�Ӧ�е���������________����ԭ����________��ÿ����1 mol Na2FeO4ת��________mol���ӡ�
�ڼ�Ҫ˵��K2FeO4��Ϊˮ������ʱ���������__________________________________
________________________________________________________________________��
(2)ʪ���Ʊ��������(K2FeO4)�ķ�Ӧ��ϵ�����������ӣ�Fe(OH)3��ClO����OH����FeO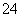 ��Cl����H2O��
��Cl����H2O��
��д������ƽʪ���Ƹ�����ط�Ӧ�����ӷ���ʽ��____________________________
________________________________________________________________________��
��ÿ����1 mol FeO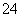 ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
�۵����£��ڸ���������Һ�м���KOH�����Ϳ������������(K2FeO4)��˵��ʲô����________________________________________________________________________��
������(1) �ٸ÷�Ӧ�еõ��ӵ������ǹ������ƣ�ʧ���ӵ������ǹ������ƺ����������������������ǹ������ƣ���ԭ���ǹ������ƺ�����������
2FeSO4 �� 6Na2O2===2Na2FeO4 �� 2Na2O �� 2Na2SO4 �� O2��ת�Ƶ���[��Դ:Z.xx.k.Com]
����������2 mol����������������������10 mol
1 mol 5 mol
����ÿ����1 mol FeO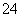 ת�Ƶ���5 mol��
ת�Ƶ���5 mol��
�ڸ�����ؾ���ǿ�����ԣ���ɱ��������������������������ԭΪFe3����Fe3��ˮ������Fe(OH)3����������ˮ���������ʶ�������
(2) �ٸ����������������������Ƿ�Ӧ����ݸ�����غ�������������Ԫ�ػ��ϼ۵ı仯֪���������ǻ�ԭ�������������õ��ӷ�Ӧ��Ԫ�صĻ��ϼ۽��ͣ��������������л��ϼ۱仯����һ��Ԫ�����ȣ��Ҵ��������������Ӧ���������������������ӷ���ʽΪ2Fe(OH)3 �� 3ClO�� �� 4OH�� === 2FeO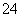 �� 3Cl�� �� 5H2O��
�� 3Cl�� �� 5H2O��
��2Fe(OH)3 �� 3ClO�� �� 4OH��===2FeO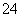 ��3Cl�� �� 5H2Oת�Ƶ���
��3Cl�� �� 5H2Oת�Ƶ���
�������������������������� 2 mol ��3 mol ��6 mol
1 mol 3 mol
���������������������������� 0.15 mol 0.3 mol
���ÿ����1 mol FeO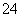 ת��3 mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ0.15 mol��
ת��3 mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ0.15 mol��
�۸��¶���K2FeO4��Na2FeO4���ܽ��С��
�𰸣�(1) ��Na2O2��Na2O2 ��FeSO4��5
�ڸ�����ؾ���ǿ�����ԣ���ɱ��������������������������ԭΪFe3����Fe3��ˮ������Fe(OH)3����������ˮ���������ʶ�����
(2) ��2Fe(OH)3 �� 3ClO�� �� 4OH�� ===2FeO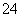 ��3Cl���� 5H2O����3��0.15 mol
��3Cl���� 5H2O����3��0.15 mol
�۸��¶���K2FeO4��Na2FeO4���ܽ��С


 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��˿��������ȼ��ʱ���ɹ۲쵽��������____________________����Ӧ�Ļ�ѧ����ʽΪ__________________________��Ȼ����ȼ�պ�ļ���ƿ�м�������ˮ�����ɹ۲쵽��Һ��________ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K�� ��Na�� ��Fe2�� ��Ba2����NH |
| ������ | OH����NO |
(1)��A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ_________________________________________________________��
��A��B��Һ��Ϻ���ȳ����ԣ���Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��AΪ___________________________________________________________��
�ھ�����������������Һ��Ƶ�ԭ����������֣�
��.________________________________________________________________________��
��.________________________________________________________________________��
������һ������֤��������Һ��Ƶ�ԭ��__________________________________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪ
______________________________________________________________________ __
__
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Cl2�Ƿ�֯��ҵ�г��õ�Ư����Na2S2O3����ΪƯ�ײ�ƥ��ġ����ȼ�����S2O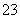 ��Cl2��Ӧ�IJ���֮һΪSO
��Cl2��Ӧ�IJ���֮һΪSO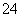 ������˵���У��������(����)
������˵���У��������(����)
A���÷�Ӧ�е���������S2O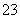
B��SO2��������Ư��ԭ����ͬ������Ҳ������SO2����֯��ҵ��Ư��
C��������Ӧ�У�ÿ����1 mol SO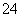 ��
�� ����ȥ2 mol Cl2
����ȥ2 mol Cl2
D�����ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O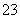 >Cl��
>Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ڱ�����Ԫ���У���Ԫ�����ҡ���������Ԫ�ؽ�������(���»�����)���ס�����Ԫ�ص�ԭ������֮�͵��ڱ�Ԫ�ص�ԭ��������������Ԫ��ԭ�ӵ�����������֮��Ϊ20������˵����ȷ����(����)
A���ס��ҡ�����������Ԫ�ؾ�Ϊ������Ԫ��
B����̬�⻯����ȶ��ԣ���>��
C������������Ӧ��ˮ��������ԣ���<��
D��Ԫ�ض������γɵĻ������п��ܺ������Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F���ֶ�����Ԫ�أ���ԭ������������������B��Cͬ���ڣ�D��E��Fͬ���ڣ�A��Dͬ���壬C��Fͬ���壬CԪ�ص�ԭ�������������Ǵ�����������������D����������ԭ�Ӱ뾶��������Ԫ�ء���֪����Ԫ�����γɵij��������ڳ��³�ѹ�������������壬�����ǹ��塣��ش��������⣺
(1)Ԫ��D�����ڱ��е�λ��__________��
(2)C��D��F����Ԫ���γɵļ����ӵİ뾶�ɴ�С��˳����(�����ӷ��ű�ʾ)______��
(3)��A��B��C����Ԫ����ԭ�Ӹ�����4��2��3�γɻ�����X��X��������ѧ��������________________________________________________________________________��
(4)��E�ǽ���Ԫ�أ��䵥������������Ӧ�����ں� �Ӹֹ죬��д����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�Ӹֹ죬��д����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
��E�Ƿǽ���Ԫ�أ��䵥���ڵ��ӹ�ҵ������ҪӦ�ã���д��������������ǿ����Һ�����ӷ���ʽ��_______________ ___________________________________________________
___________________________________________________
_________________________________ _______________________________________��
_______________________________________��
(5)FC2�����ж����ŷŵ����������γ����꣬д��FC2��������ˮ������Ӧ�Ļ�ѧ����ʽ_____________________________________________________________________________
_______________ _________________________________________________________��
_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ϵͳ������������������
(1) 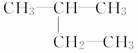 ______________________________________________��
______________________________________________��
(2) 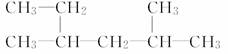 ___________________________________��
___________________________________��
(3) 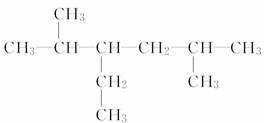 _______________________________��
_______________________________��
(4) 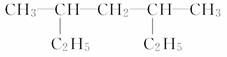 ___________________________________��
___________________________________��
(5) 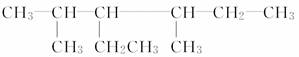 __________________________��
__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��Һ�����п��ܴ��ڵ������У�Na+��Ag+��Ba2+��Fe3+��Al3+��AlO2-��
S2-��SO32-��CO32-��SO42-����ȡ����Һ����ʵ�飬ʵ��������:
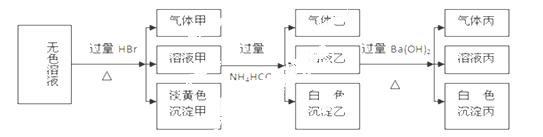
�Իش��������⣺
��1�����ɳ��������ӷ���ʽΪ�� ��
��2������Һ�����ɳ����ҵ����ӷ���ʽΪ ��
��3����֪��������������������(������ˮ������HBr)���������ʵ����һ���������ֳɷ֣��������Լ��Լ����������±�(����ÿһ�ж�Ӧ��ȷ���ɵ÷�)
| ���� | ʵ��Ŀ�� | �Լ� | ���� |
| 1 | |||
| 2 | |||
| 3 | ����CO2 | ����ʯ��ˮ | ��Һ����� |
��4����������������Һ�϶����ڵ������У� ��
��5�������Һ�п��ܴ��ڵ����ӣ���ʵ��֤���Ƿ���ڵķ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com