
ij�������ǽ�������K�����ַǽ���Ԫ����ɣ�����һ�ֳ�Ӳ���ʣ�������ĥ����ʴ�������ȳ���������������ԣ���������ѧ��ѧ�еij�������Ϊԭ���������ģ�����������������ʾ���������߿��ڵ�ת����Ϊ̽��C����ɶ��裬G��F��H��������ˮ����Ϊ��ɫ��ĩ��ͼ�г�M��K(����AԪ��)��������ĸ�����ľ�Ϊ��ѧ��ѧ�������ʣ�
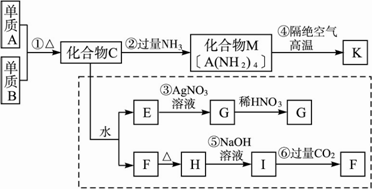
(1)д����ѧʽ������B________��������F________��
(2)д�����з�Ӧ�����ӷ�Ӧ����ʽ����________����________��
(3)��Ӧ�ܵĻ�ѧ��Ӧ����ʽΪ________��
|
�����𰸣�(1)Cl2��H2SiO3��(2)Ag+��Cl�� ����˼·��������E�м�����������Һ������ɫ�������Ҵ˳���������ϡ���ᣬ��˵��������C��Ӧ����Ԫ�ش��ڣ��ֻ�����C��ˮ��Ӧ�������F������ˮ��ͬʱ����Ⱥ����ò���Ҳ������ˮ�����ܹ���NaOH��Һ��Ӧ����ɽ�һ��˵��������C�к��й�Ԫ��(������Ӧ���Ȼ����ܹ�����ˮ)���ֻ�����K���ɷǽ����γɵij�Ӳ���ʣ���AΪSi��BΪCl2��CΪSiCl4��FΪH2SiO3��KΪSi3N4�� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
(1)д����ѧʽ������B��__________��������F��__________��
(2)����H��__________���塣
(3)д�����з�Ӧ�����ӷ���ʽ��
��Ӧ��______________________________��
��Ӧ��______________________________��
��Ӧ��______________________________��
(4)д����Ӧ�ںܵ͢Ļ�ѧ����ʽ��
��___________________________________��
��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

������������⡣
��1��ָ��K���������ľ�������_________��
��2��д����ѧʽ������B_________��������F_________��
��3��д�����з�Ӧ�����ӷ���ʽ��
��Ӧ��___________________________;
��Ӧ��___________________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ?
?
��1��д����ѧʽ������B___________��������F___________��?
��2��д�����з�Ӧ�����ӷ�Ӧ����ʽ����_________����__________��?
��3����Ӧ�ܵĻ�ѧ��Ӧ����ʽΪ____________��
?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 4.3 ���ϲ��ϵ�������ϰ���������棩 ���ͣ������
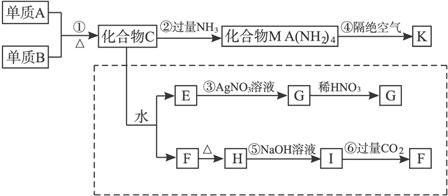
ij�������ǽ�������A�������ַǽ���Ԫ����ɣ���������Ԫ�صĻ��ϼ�Ϊ��������ۻ�����ۣ�����һ�ֳ�Ӳ���ʣ�������ĥ����ʴ�������ȳ������������������A���ɻ�����B�����з�Ӧ�Ƶã���B��NH3�D��C[C�Ļ�ѧʽΪSi(NH2)4]����C�������������·ֽ�õ�A��Ϊ̽��B����ɣ���������ͼ��ʾ��ת��ʵ�飬ͼ��G��F��H��Ϊ������ˮ�����ʣ���Ϊ��ɫ��ĩ��ͼ����ĸ�����ľ�Ϊ��ѧ��ѧ���������ʡ�

��ش��������⣺
(1)д��������B�ͻ�����G�Ļ�ѧʽ__________��________��
(2)д����Ӧ�ڵ����ӷ���ʽ�� ___________________________________________��
(3)д��C�������������·ֽ�õ�A�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)������Ӧ�ۣ����ܵó��Ľ�����________________________________________(��������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com