
| ���� ���� | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| �ܽ�ȣ�mol•L-1�� | 1.34��10-6 | 7.1��10-7 | 1.1��10-8 | 6.5��10-5 | 1.0��10-6 |
���� ��1���ٸ���c�����⣩=$\frac{c������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
�ڵζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ�����
��2���ٸ��ݸ��������Һ����ǿ������ѡ��ζ������ͣ�
�ڸ��ݸ�������������ȥ���ϴ��һ�飬����ƽ��ֵ����Ϸ�Ӧ�����ӷ���ʽ���㣻
��3���ζ�����ʱ�������μӵζ������ζ�����ָʾ����Ӧ������������ɫ�仯�ij�������֤�ζ����ͱ��ζ�����ȫ��Ӧ��
��� �⣺��1����A��ʢװδ֪Һ����ƿ��������ˮϴ��������δ֪Һ��ϴ������δ֪Һƫ�࣬���ĵı�Һ���ƫ������ƫ��A��ȷ��
B���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ���������ĵı�Һ�������ƫС���ⶨ���ƫ�ͣ���B����
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ����������Ũ��ƫ�ͣ����ƫ�ⶨ���ƫ��C��ȷ��
D���ζ�ǰ����ʽ�ζ��ܼ��������ݣ��ζ��������ݣ��������ƫС���ⶨ���ƫ�ͣ���D����
�ʴ�Ϊ��AC��
�ڵζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ���������˳��ӦΪECBADGF���ʴ�Ϊ��ECBADGF��
��2���ٸ�����ؾ��и�ʴ�ԣ��ɸ�ʴ��Ӧ����ʽ�ζ��ܣ��ʴ�Ϊ����ʽ��
�ڸ��ݱ����ṩ�����ݿ�֪��20.60mL������ǰ��3������ƫ��ϴ�ΪżȻ��Ӧ��ȥ��������ʵ������ȥ�ĸ��������Һ�����Ϊ$\frac{19.90+20.00+20.10}{3}$mL=20.0mL���������ص����ʵ���Ϊ1.000��10-2mol•L-1��0.02L=2.000��10-4mol������5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��֪�������ӵ����ʵ���Ϊ1.000��10-3mol������5.000g��ˮ���������ӵ����ʵ���Ϊ$\frac{100mL}{25.00mL}$��1.000��10-3mol=4.000��10-3mol��������Ϊ0.224g��������Ԫ�ص���������=$\frac{0.224}{5.000}$��100%=4.480%��
�ʴ�Ϊ��4.480%��
��3������AgNO3ȥ�ζ�NaSCN��Һ����ѡ�õĵζ�ָʾ�������ʵ��ܽ��Ӧ��AgSCN�����������ԣ�ӦΪNa2CrO4�����������ש��ɫ�������ɣ��ʴ�Ϊ��C��
���� ���⿼�����ʵĺ����IJⶨ��Ϊ��Ƶ���㣬�漰����к͵ζ�������ע�������к͵ζ��IJ���������ָʾ����ѡ�����������������е��Ѷȵ����⣬ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ʵ����Ҫ����0��lmol/LNaOH��Һ400mL�����ȡNaOH ����1.6g | |
| B�� | ��������ͷ���ijЩ����Ԫ�ػ�ѧ���ʵ�չ�� | |
| C�� | Al2O3�۵�ܸߣ��������ͻ���� | |
| D�� | ��п���������Ӻ���ͬһϡ�����У�п���Ϸ���������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
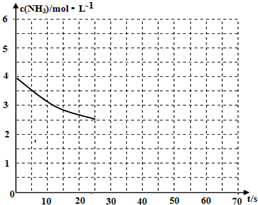 ��2�����ð�����ȡ����[CO��NH2��]�ķ�ӦΪ��2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g����H��0��
��2�����ð�����ȡ����[CO��NH2��]�ķ�ӦΪ��2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g����H��0���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 1000 | 1100 |
| ƽ�ⳣ�� | 0.68 | 0.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ���ش��������⣺
�£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
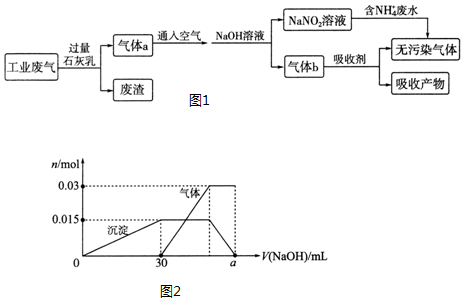
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com