
����Ŀ��������Һ��Ũ�ȹ�ϵ��ȷ����(����)
A. С�մ���Һ�У�c(Na��)��c(H��)��c(HCO)��c(CO![]() )��c(OH��)
)��c(OH��)
B. CH3COONa��Һ�У�c(CH3COO��)>c(Na��)
C. ���ʵ���Ũ����ȵ�CH3COOH��Һ��CH3COONa��Һ�������ϣ�c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH)
D. 0.1 mol/L��NaHA��Һ����pH��4����c(HA��)>c(H��)>c(H2A)>c(A2��)
���𰸡�C
��������A.��ɲ��غ㣬����B. CH3COONa��Һ�У����������Ҫˮ�⣬c(CH3COO��)��c(Na��)������C. ���ʵ���Ũ����ȵ�CH3COOH��Һ��CH3COONa��Һ�������ϣ��������غ㣺2c(Na��)��c(CH3COO��)��c(CH3COOH)�͵���غ���c(Na��)��c(H��)��c c(CH3COO��)��c(OH��)����ʽ���е���c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH)��C��ȷ��D. 0.1 mol/L��NaHA��Һ����pH��4��˵��HA���ĵ������ˮ�⣬��c(HA��)>c(H��)> c(A2��)> c(H2A)��D������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
������ | CO |
������ | Al3����Fe3����Mg2����NH |
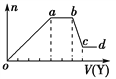
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ����(V)�Ĺ�ϵ��ͼ��ʾ��
��1����Y�����ᣬ��oa��ת��Ϊ����������(ָ��Դ��X��Һ�ģ���ͬ)��________�� bc�η�����Ӧ�����ӷ���ʽ��________________________��
��2����Y��NaOH��Һ����X��һ�����е�������______________________��bc�η�����Ӧ�����ӷ���ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ����һ����Ũ���ᣬ����NO2��N2O4��NO�Ļ�����壬��Щ��������1.12 L O2(��״��)��Ϻ�ͨ��ˮ�У����屻ˮ��ȫ���ա�����ԭ������Һ�м���5 mol��L��1 H2SO4��Һ100 mL��������ܽ��Cu������Ϊ(����)
A. 6.4 g B. 9.6 g C. 19.2 g D. 24 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������밲ȫ�¹ʴ�����ȷ���ǣ� ��
A.��H2��ԭCuO��ʵ��ʱ��Ϊ��ֹH2��ը��ʵ����ϣ�Ӧ��ֹͣͨH2 �� ��ֹͣ����
B.����ϡ������Һʱ��������Ͳ����һ�������ˮ��������������������Ũ���ᣬ�����Ͻ���
C.���Թܼд��Թܵ��������ϼ�ס����ܿ�Լ ![]() �����ֳ��Թܼг���ĩ�ˣ����м���
�����ֳ��Թܼг���ĩ�ˣ����м���
D.��Һ©����ʹ��ǰҪ��©��ʹ�ù�����Ҫ�ʵ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڿ��淴Ӧ3H2(g) + N2(g)=2NH3(g)�����д�ʩ��ʹ��Ӧ���л���Ӱٷ�������ѧ��Ӧ���ʺͻ�ѧƽ�ⳣ�����仯����
A. ����ѹǿ B. �������N2 C. ʹ�ø�Ч���� D. �����¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����pH=1����ɫ��Һ���ܴ����������������
A.NH4+��Mg2+��SO42-��Cl-B.Ba2+��K+��OH-��NO3-
C.Al3+��Cu2+��SO42-��Cl-D.Na+��Ca2+��Cl-��AlO2-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
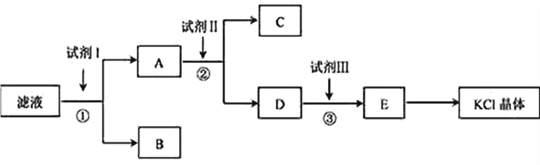
����Ŀ���Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����
�ش��������⣺
��1����ʼ��Һ��pH_______7������ڡ�����С�ڡ����ڡ�������ԭ����______________��
��2���Լ�I�Ļ�ѧʽΪ________�����з�����Ӧ�����ӷ���ʽΪ_________________________��
��3���Լ���Ļ�ѧʽΪ___________�����м����Լ����Ŀ����__________________________��
��4���Լ����������___________�����з�����Ӧ�����ӷ���ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭ�Ӻ�������δ�ɶԵ��ӵ���������ȷ����
A. ������ͬB. ��������ͬ
C. ��������״��ͬD. ��ͬһ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������仯���ǣ� ��
A. ú�ĸ��� B. ʯ�͵��ѽ� C. ú������ D. ʯ�͵ķ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com