
����Ŀ��������ͭ[Cux(Met)y��Met��ʾ�����������]��һ�������������Ӽ���Ϊȷ��������ͭ[Cux(Met)y]����ɣ���������ʵ�飺
(1)��ȡһ����������Ʒ����ƿ�У���������������ˮ��ϡ���ᣬ������ȫ���ܽ⣬��ȴ����Һ�ֳ����ȷݡ�
��ȡ����һ����Һ��������ҺpH��6��8֮�䡣����0.1000 mol/LI2�ı���Һ25.00 mL,��ַ�Ӧ�����2��3��ָʾ��X����0.1000 mol/LNa2S2O3����Һ�ζ�����ɫǡ����ȥ��������Ӧ��![]() ������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������һ����Һ�м���NH3��H2O-NH4Cl������Һ��������70�����ң�����2-3��ָʾ��PAN����0.02500 mol/LEDTA (Na2H2Y)����Һ�ζ�����Cu2+(���ӷ���ʽΪCu2++H2Y2--=CuY2-+2H+)������EDTA����Һ28.00 mL��
(1)ָʾ��XΪ ____��
(2)��Na2S2O3��Һ�ζ�ʱ����pH��С������S��SO2���ɡ�д��S2O32-��H+��Ӧ�����ӷ���ʽ ___________ ��
(3)���ζ���ˮϴ��δ��EDTA����Һ��ϴ�����Cu2+�����ʵ�����____(�ƫ����ƫС�����䡱)��
(4)ͨ������ȷ��������ͭ[Cux(Met)y]�Ļ�ѧʽ(д���������)________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�������������
I.�������������
��14C ��16O ��14N ��18O ��O2 ��O3
��CH3-CH3 ��C2H6 ��CH3- CH2-OH �� CH3-O-CH3
(1)��Ϊͬλ�ص���______��(����ţ���ͬ)��������ͬ��������ͬ����______________
(2)��Ϊͬ�����������_________, ��Ϊͬ���칹�����_____________________
II.��25 C��101 kPa�������£�

���ڷ�ӦH2(g)+Cl2(g)= 2HCl(g), �������2 mol HCI(g)ʱ,��Ӧ�����зų�183 kJ����������Ͽ�1 mol H- Cl�������������____ kJ.

III.��ͼ��ʾ��ԭ���װ���У������ĵ缫��Ӧ����ʽ��________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦBr��H2![]() HBr��H�������仯��ͼ��ʾ������ͼ���ж�����˵����ȷ���ǣ� ��
HBr��H�������仯��ͼ��ʾ������ͼ���ж�����˵����ȷ���ǣ� ��
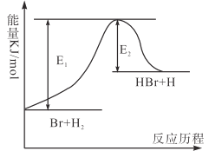
A.HBr��������H2��������
B.��Ӧ2C��O2��2CO�������仯���̣����ͼ�ı仯��������
C.���÷�Ӧ��ʼʱ�����������Ӧ�ﵽƽ��ʱ���յ���������
D.�÷�Ӧ�У�H��H���������յ�����������H��Br���γ��ͷŵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���������ڿ������ܱ������������������ơ�
I. ��ͬѧ������ַ���������![]() �Ƿ������ʡ�
�Ƿ������ʡ�
����һ��ȡ��������ϡ�������������ɣ���Ϊ��������û�б��ʡ�
��������ȡ���������Ȼ�����Һ�а�ɫ�������ɣ���Ϊ����������ȫ���ʡ�
������������������ۣ�
����һ��_________(���������������)��������_____________________��
��������_________(���������������)��������____________________��
��. ��ͬѧΪ��̽��![]() �Ƿ������ʣ��������ʵ��ⶨ
�Ƿ������ʣ��������ʵ��ⶨ![]() ��Һ��ʵ��Ũ�ȡ�
��Һ��ʵ��Ũ�ȡ�

(1)�ٷ�Һ©����Ӧ����������________(����ĸ)��
a.Ũ���� b.65%���� c.Ũ����
��װ��B��ʢװ���Լ�Ϊ_____________��
(2)ʵ��ǰ����װ��C����3.2g����![]() ��Һʵ�ʵ����ʵ���Ũ��Ϊ___________________(����2λ��Ч����)��
��Һʵ�ʵ����ʵ���Ũ��Ϊ___________________(����2λ��Ч����)��
(3)��ʵ��װ�û�����һ�����Ե�ȱ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѪҺ��pHͨ����7.35-7.45֮���ԭ����ѪҺ�д���NaH2PO4-Na2HPO4�Ȼ�����ϵ�������£�Ka1(H3PO4)=7.6��10-3��Ka2(H3PO4)=6.3��10-8������ָ����Һ�������ʵ���Ũ�ȹ�ϵ��ȷ����
A.0.1mol/L NaH2PO4��Һ��2c(HPO42-)+3c(PO43-)��c(Na+)-c(H2PO4-)
B.�����£�pH=7��NaH2PO4��Na2HPO4�Ļ����Һ��c(Na+)��c(HPO42-)��c(H2PO4-)
C.��10 mL0.1mol/L NaH2PO4��Һ�м���5mL 0.4 mol/L NaOH��Һ��c(H+)+3c(H3PO4)+2c(H2PO4-)+c(HPO42-)=c(OH-)
D.���ʵ���Ũ�����NaH2PO4��Na2HPO4��Һ�������ϣ�3[c(H2PO4-)+c(HPO42-)+c(PO43-)]=2c(Na+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2O2����ȡ��������ˮ���������Ӧ���ǵ�ǰ��ѧ�о����ȵ㡣
(1)����������ԭ������ȡH2O2��ԭ������ͼ��ʾ��
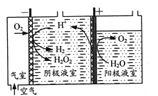
�������淢���ĵ缫��Ӧ�У�
��.2H++O2+2e-=H2O2
��. H2O2+2H++ 2e-=2H2O
��. 2H+ +2e-=H2��
��д����������ĵ缫��Ӧʽ��___��
������������ͬʱ����ͬ��ʼpH(��С��2)�����£�H2O2Ũ������ʱ��ı仯��ͼ��ʾ��c(H+)������С��������H2O2��ȡ��ԭ����_______��
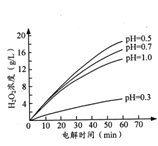
(2)����������£�H2O2��һ�ִ��ֽ�������£�
H2O2(aq)+Mn2+(aq)=��OH(aq)+Mn3+(aq)+OH-(aq) H=akJ/mol
H2O2(aq)+ Mn3+(aq) +2OH-(aq)= Mn2+(aq) +��O2-(aq) +2H2O(l) H=bkJ/mol
��OH(aq) +��O2-(aq)=O2(g) +OH-(aq) H=ckJ/mol
2H2O2(aq)= 2H2O(l)+O2(g) ��H=_______ ���÷�Ӧ�Ĵ���Ϊ ____��
(3)H2O2��O3��ˮ�п��γɾ��г�ǿ�����������ǻ����ɻ�(��OH)������Чȥ����ˮ�еĴ����������(H2PO2-)��
�������������¡�OH��H2PO2-������PO43-��������l.7g��OH�ɴ�����0.001mol/L H2PO2-��ģ���ˮ�����Ϊ______��
��Ϊ�Ƚϲ�ͬͶ�Ϸ�ʽ�º�H2PO2-ģ���ˮ�Ĵ���Ч���������ݵ������ˮ��Ʒ�м������H2O2��O3������һ���ټ���FeSO4����Ӧ��ͬʱ�䣬ʵ������ͼ��ʾ��
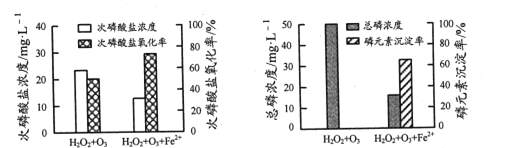
����FeSO4�������������ʡ���Ԫ�س����ʾ�������ߣ�ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Դ���ճ�����߿Ƽ������ж��й㷺Ӧ�á�����˵������ȷ����(����)
A.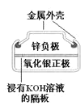 �����ĵ缫��ӦʽΪAg2O��2e-��H2O=2Ag��2OH-
�����ĵ缫��ӦʽΪAg2O��2e-��H2O=2Ag��2OH-
B. пͲ������������������Ӧ��пͲ��䱡
пͲ������������������Ӧ��пͲ��䱡
C.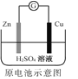 Zn2+��Cu�缫�����ƶ���Cu�缫������Һ��H+Ũ������
Zn2+��Cu�缫�����ƶ���Cu�缫������Һ��H+Ũ������
D.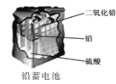 ʹ��һ��ʱ��������Һ�����Լ��������������½�
ʹ��һ��ʱ��������Һ�����Լ��������������½�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. 24 g þ��27 g���У�������ͬ��������
B. ͬ�������������ͳ����У���������ͬ
C. 1 mol��ˮ��1 molˮ�У���������Ϊ2��1
D. 1 mol�����1 mol��ϩ�У���ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ�������Τ(Remdesivir)������״������Ⱦ����DZ�ڵ�����Ч����KΪҩ��ϳɵ��м��壬��ϳ�·����ͼ��ʾ��
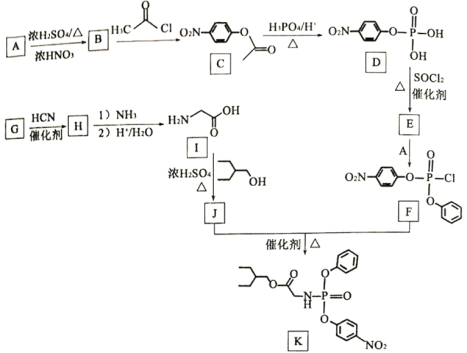
��֪������Ϣ
��![]()
��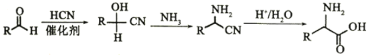
�ش��������⣺
��1��B�Ļ�ѧ����Ϊ____________��
��2��J�к��й����ŵ�����Ϊ____________��
��3����B����C�ķ�Ӧ����Ϊ____________��
��4����G����H�Ļ�ѧ��Ӧ����ʽ____________��
��5��E�к�����Clԭ�ӣ���E�Ľṹ��ʽ____________��
��6��X��C��ͬ���칹�壬д������һ����������������X�Ľṹ��ʽ____________��
�ٱ����Ϻ��������ұ�����ֻ��һ����ԭ�ӣ�
����FeCl3��Һ������ɫ��Ӧ��
��1mol��X����������Na��Ӧ������2gH2��
��7������ɱ��״�(![]() )Ϊԭ�Ϻϳɻ�����
)Ϊԭ�Ϻϳɻ�����![]() ��·��____________(�����Լ���ѡ)��
��·��____________(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com