
 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KW����ҺŨ�ȵĸı���ı� |
| B��Kspֻ�����ܵ���ʵ����ʺ��¶��йأ�������Һ�е�����Ũ���� |
| C�����Ѵﵽ��ѧƽ��ķ�Ӧ���ı�ѹǿ��ƽ�ⳣ��(K)һ���ı� |
| D��һ������£�һԪ����HA��Ka Խ�������������Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��10��1 | B��1��9 | C��9��2 | D��9��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3COONa��Na2CO3��NaHSO4��NaCl | B��HCl��CH3COOH��NH4Cl��NaHCO3 |
| C��NaOH��Ba(OH)2��H2SO4��HCl | D��NH4Cl��CH3COOH��NaHSO4��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3CH2OH(l) + 3O2 (g) = 2CO2 (g) + 3H2O(l)��H =��a kJ/mol |
| B��CH4 (g) + 2O2 (g) = 2H2O(g) + CO2 (g)��H =��b kJ/mol |
C��H2 (g) +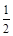 O2 (g) = H2O(l)��H = c kJ/mol O2 (g) = H2O(l)��H = c kJ/mol |
| D��2CH��CH(g) + 5O2(g) =4CO2 (g) + 2H2O(l)��H =��d kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | ����Һ�����mL�� | �����������Һ�������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.70 |
| 2 | 20.00 | 6.00 | 26.00 |
| 3 | 20.00 | 1.40 | 21.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���� | HCN | HF | CH3COOH | HNO2 |
| ���볣�� | 6.2��10��10 | 6.8��10��4 | 1.7��10��5 | 6.4��10��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��������Һ | ������������Һ | �۴�����Һ | ������ |
HSO4��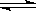 H+��SO42�� H+��SO42�� | HSO4��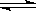 H+��SO42�� H+��SO42�� | CH3COOH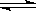 CH3COO��+H+ CH3COO��+H+ | HCl = H+��Cl�� |
| 10�G | 29�G | 1.33�G | 100�G |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com