
(9��)��ҵ�ϲ��õ�һ����ˮ�����������£�������ˮ��pH��5.0 ~ 6.0֮�䣬ͨ���������Fe(OH)3��Fe(OH)3�������ԣ�������������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ(��Ʋ��)�����㣬�����˸�ѡ���������ã�ij����С���ø�ԭ��������ˮ�����װ��ʾ��ͼ��ͼ��ʾ��
(l)ʵ��ʱ����ˮ������Ũ�Ƚ�С�����������ϲ�����������ʻ�������ʹ�������γɸ�������ʱӦ����ˮ�м���������
a��HCl b��CH3CH2OH c��Na2SO4 d�� NaOH
(2)��������ʵ�ʷ����������缫��Ӧ������һ����Ӧ����һ����ɫ���壬�������ĵ缫��Ӧʽ�ֱ���I�� �� II��
(3)��ȼ�ϵ����������̼����Ϊ����ʣ�CH4Ϊȼ�ϣ�����Ϊ��������ϡ�������������缫�������ĵ缫��Ӧ��__________________________________��
(4)��֪ȼ�ϵ������1.6 g CH4�μӷ�Ӧ����C�缫�������������� L (��״��)��
(5)����װ���еļײ��ֻ�Ϊ��ͼ��ʾ��װ�ã�
�����CuSO4��ȫ��Ӧ������������� ���ָܻ���ԭŨ�ȡ�
 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ����и߶���������Ի�ѧ�Ծ����������� ���ͣ������
(9��)��ҵ���Ի�����Ϊԭ���������ᣬ������Ҫ��һ���Ǵ�����(�����б��ֺ��º�������)��2SO2(g)��O2(g)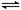 2SO3(g) ��H����196.6 kJ��mol��1
2SO3(g) ��H����196.6 kJ��mol��1
(1)������Ϊ��߷�Ӧ���ʺ�SO2��ת���ʣ����д�ʩ���е��� ��
A����װ���г���O2 B�������¶�
C����װ���г���N2 D����װ���г��������SO2
(2)���º�ѹ��ͨ��3mol SO2��2mol O2 �����������ƽ��ʱ�������������Ϊ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ 5mol SO2(g)��3.5 mol O2(g)��1mol SO3(g)������˵����ȷ����
A����һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9kJ
B������ƽ��SO2��ת�������
C������ƽ��ʱ��O2����������
D���ڶ���ƽ��ʱSO3�������������2/9
(3)500 ��ʱ��10 mol SO2��5.0 mol O2�������Ϊ1�̵ĺ����ܱ������У�SO2ת��ΪSO3��ƽ��ת����Ϊ0.95����500��ʱ��ƽ�ⳣ��K= ��
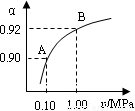
(4)550 �棬A��B��ʾ��ͬѹǿ�µ�ƽ��ת����(��ͼ)��ͨ����ҵ�����в��ó�ѹ��ԭ���� ��
���Ƚϲ�ͬѹǿ�µ�ƽ�ⳣ����K(0.10 MPa) K(1.0 MPa)��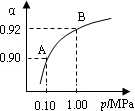
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ������ѧ����ѧ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
(9��)��ҵ�ϲ��õ�һ����ˮ�����������£�������ˮ��pH��5.0 ~ 6.0֮�䣬ͨ���������Fe(OH)3��Fe(OH)3�������ԣ�������������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ(��Ʋ��)�����㣬�����˸�ѡ���������ã�ij����С���ø�ԭ��������ˮ�����װ��ʾ��ͼ��ͼ��ʾ��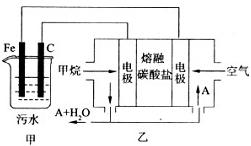
(l)ʵ��ʱ����ˮ������Ũ�Ƚ�С�����������ϲ�����������ʻ�������ʹ�������γɸ�������ʱӦ����ˮ�м���������
a��HCl b��CH3CH2OH c��Na2SO4 d�� NaOH
(2)��������ʵ�ʷ����������缫��Ӧ������һ����Ӧ����һ����ɫ���壬�������ĵ缫��Ӧʽ�ֱ���I��  �� II��
�� II��
(3)��ȼ�ϵ����������̼����Ϊ����ʣ�CH4Ϊȼ�ϣ�����Ϊ��������ϡ�������������缫�������ĵ缫��Ӧ��__________________________________��
(4)��֪ȼ�ϵ������1.6 g CH4�μӷ�Ӧ����C�缫�������������� L (��״��)��
(5)����װ���еļײ��ֻ�Ϊ��ͼ��ʾ��װ�ã�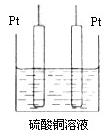
�����CuSO4��ȫ��Ӧ������������� ���ָܻ���ԭŨ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�߶���������Ի�ѧ�Ծ��������棩 ���ͣ������
(9��)��ҵ���Ի�����Ϊԭ���������ᣬ������Ҫ��һ���Ǵ�����(�����б��ֺ��º�������)��2SO2(g)��O2(g) 2SO3(g)
��H����196.6 kJ��mol��1
2SO3(g)
��H����196.6 kJ��mol��1
(1)������Ϊ��߷�Ӧ���ʺ�SO2��ת���ʣ����д�ʩ���е��� ��
A����װ���г���O2 B�������¶�
C����װ���г���N2 D����װ���г��������SO2
(2)���º�ѹ��ͨ��3mol SO2 ��2mol O2 �����������ƽ��ʱ�������������Ϊ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ 5mol SO2(g)��3.5 mol O2(g)��1mol SO3(g)������˵����ȷ����
A����һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9kJ
B������ƽ��SO2��ת�������
C������ƽ��ʱ��O2����������
D���ڶ���ƽ��ʱSO3�������������2/9
(3)500 ��ʱ��10 mol SO2��5.0 mol O2�������Ϊ1�̵ĺ����ܱ������У�SO2ת��ΪSO3��ƽ��ת����Ϊ0.95����500��ʱ��ƽ�ⳣ��K= ��
(4)550 �棬A��B��ʾ��ͬѹǿ�µ�ƽ��ת����(��ͼ)��ͨ����ҵ�����в��ó�ѹ��ԭ���� ��
���Ƚϲ�ͬѹǿ�µ�ƽ�ⳣ����K(0.10 MPa) K(1.0 MPa)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
(9��)��ҵ�ϲ��õ�һ����ˮ�����������£�������ˮ��pH��5.0 ~ 6.0֮�䣬ͨ���������Fe(OH)3��Fe(OH)3�������ԣ�������������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ(��Ʋ��)�����㣬�����˸�ѡ���������ã�ij����С���ø�ԭ��������ˮ�����װ��ʾ��ͼ��ͼ��ʾ��

(l)ʵ��ʱ����ˮ������Ũ�Ƚ�С�����������ϲ�����������ʻ�������ʹ�������γɸ�������ʱӦ����ˮ�м���������
a��HCl b��CH3CH2OH c��Na2SO4 d�� NaOH
(2)��������ʵ�ʷ����������缫��Ӧ������һ����Ӧ����һ����ɫ���壬�������ĵ缫��Ӧʽ�ֱ���I�� �� II��
(3)��ȼ�ϵ����������̼����Ϊ����ʣ�CH4Ϊȼ�ϣ�����Ϊ��������ϡ�������������缫�������ĵ缫��Ӧ��__________________________________��
(4)��֪ȼ�ϵ������1.6 g CH4�μӷ�Ӧ����C�缫�������������� L (��״��)��
(5)����װ���еļײ��ֻ�Ϊ��ͼ��ʾ��װ�ã�
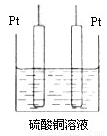
�����CuSO4��ȫ��Ӧ������������� ���ָܻ���ԭŨ�ȡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com