
 µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŌŖĖŲ£¬µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ£®
µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŌŖĖŲ£¬µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ£®·ÖĪö £Ø1£©øł¾ŻĻõĖįļ§ČÜŅŗÖŠļ§Ąė×ÓĖ®½ā“Ł½ųĮĖĖ®µÄµēĄė£¬ĒāŃõ»ÆÄĘČÜŅŗÖŠĒāŃõøłĄė×ÓŅÖÖĘĮĖĖ®µÄµēĄėÅŠ¶Ļ£»
£Ø2£©øł¾Ż2c£ØNH4+£©£¾c£ØNO3-£©ÅŠ¶Ļļ§Ąė×ÓÓėÄĘĄė×ÓÅØ¶Č¹ŲĻµ£¬Č»ŗóøł¾ŻČÜŅŗĻŌŹ¾¼īŠŌÅŠ¶ĻČÜŅŗÖŠø÷Ąė×ÓÅØ¶Č“óŠ”¹ŲĻµ£»
£Ø3£©øł¾Żn=$\frac{m}{M}$¼ĘĖć16gN2H4µÄĪļÖŹµÄĮ棬ŌŁøł¾ŻČČ»Æѧ·½³ĢŹ½ŹéŠ“ŌŌņŹéŠ“ČČ»Æѧ·½³ĢŹ½£»
£Ø4£©øł¾ŻÄÜĮæ±ä»ÆĶ¼£¬·“Ó¦ČȵČÓŚÕż·“Ó¦µÄ»ī»ÆÄܼõČ„Äę·“Ó¦µÄ»ī»ÆÄÜ£»¾ŻøĒĖ¹¶ØĀɼ°ŅŃÖŖµÄČżøö»Æѧ·½³ĢŹ½¾ĶæÉŅŌĒó³ö·“Ó¦µÄģŹ±ä£®
½ā“š ½ā£ŗ£Ø1£©ĻõĖįļ§ČÜŅŗÖŠ£¬Ó¦ÓĆļ§Ąė×Ó½įŗĻĖ®µēĄėµÄĒāŃõøłĄė×Ó£¬“Ł½ųĮĖĖ®µÄµēĄė£¬¶ųĒāŃõ»ÆÄĘČÜŅŗÖŠĒāŃõøłĄė×ÓŅÖÖĘĮĖĖ®µÄµēĄė£¬ĖłŅŌĻõĖįļ§ČÜŅŗÖŠĖ®µÄµēĄė³Ģ¶Č“óÓŚĒāŃõ»ÆÄĘČÜŅŗÖŠĖ®µÄµēĄė³Ģ¶Č£¬
¹Ź“š°øĪŖ£ŗ“óÓŚ£»
£Ø2£©»ģŗĻČÜŅŗÖŠ2c£ØNH4+£©£¾c£ØNO3-£©£¬c£ØNH4+£©£¾$\frac{1}{2}$c£ØNO3-£©=c£ØNa+£©£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅØ¶Č¹ŲĻµĪŖ£ŗc£ØNO3-£©£¾c£ØNH4+£©£¾c£ØNa+£©£¾c£ØOH-£©£¾c£ØH+£©£¬
¹Ź“š°øĪŖ£ŗc£ØNO3-£©£¾c£ØNH4+£©£¾c£ØNa+£©£¾c£ØOH-£©£¾c£ØH+£©£»
£Ø3£©16gN2H4µÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{16g}{32g/mol}$=0.5mol£¬Óė¶žŃõ»ÆµŖ·“Ӧɜ³ÉµŖĘųÓėĘųĢ¬Ė®·Å³ö284kJµÄČČĮ棬Ōņ1molĘųĢåėĀĶźČ«Č¼ÉÕÉś³ÉĘųĢ¬Ė®·Å³öµÄČČĮæĪŖ568kJ£¬ĖłŅŌøĆČČ»Æѧ·½³ĢŹ½ŹĒ£ŗ2N2H4£Øg£©+2NO2£Øg£©ØT3N2£Øg£©+4H2O£Øg£©”÷H=-1136kJ•mol-1£¬
¹Ź“š°øĪŖ£ŗ2N2H4£Øg£©+2NO2£Øg£©=3N2£Øg£©+2H2O £Øg£©”÷H=-1136kJ/mol£»
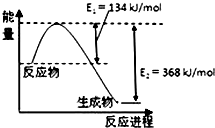
£Ø4£©øĆ·“Ó¦µÄģŹ±ä”÷H=E1-E2=134KJ/mol-368KJ/mol=-234KJ/mol£¬ĖłŅŌČČ»Æѧ·½³ĢŹ½ĪŖ£ŗNO2£Øg£©+CO£Øg£©=CO2£Øg£©+NO£Øg£©”÷H=-234kJ•mol-1£¬
N2£Øg£©+O2£Øg£©=2NO£Øg£©”÷H1=+180kJ•mol-1 ¢Ł
2NO£Øg£©+O2£Øg£©=2NO2£Øg£©”÷H=-112.3kJ•mol-1 ¢Ś
NO2£Øg£©+CO£Øg£©=CO2£Øg£©+NO£Øg£©”÷H=-234kJ•mol-1 ¢Ū
øł¾ŻøĒĖ¹¶ØĀÉ£¬¢Ś+¢Ū”Į2-¢Ł£¬µĆ»Æѧ·½³ĢŹ½ĪŖ£ŗ2NO£Øg£©+2CO£Øg£©?N2£Øg£©+2CO2£Øg£©”÷H=£Ø-112.3kJ•mol-1 £©+£Ø-234kJ•mol-1£©”Į2-£Ø+180kJ•mol-1£©=-760.3kJ/mol£¬
¹Ź“š°øĪŖ£ŗ-760.3kJ/mol£®
µćĘĄ ±¾Ģāæ¼²éĮĖĖ®µÄµēĄė”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“”¢Ąė×ÓÅØ¶Č“óŠ”±Č½ĻµČÖŖŹ¶£¬ĢāÄæÄѶČÖŠµČ£¬Éę¼°µÄÖŖŹ¶µć½Ļ¶ą£¬×¢ŅāøĒĖ¹¶ØĀÉŌŚČČ»Æѧ·½³ĢŹ½ÖŠµÄÓ¦ÓĆ£¬ŹŌĢāÅąŃųĮĖѧɜĮé»īÓ¦ÓĆĖłŃ§ÖŖŹ¶µÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | BeCl2 | B£® | CO2 | C£® | HCl | D£® | N2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
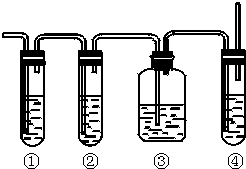
| ²½Öč1£ŗĻņ2mL 0.005mol•L-1 AgNO3ČÜŅŗÖŠ¼ÓČė2mL 0.005mol•L-1KSCNČÜŅŗ£¬¾²ÖĆ£® | ³öĻÖ°×É«³Įµķ£® |
| ²½Öč2£ŗČ”1mLÉĻ²ćĒåŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó1µĪ2mol•L-1Fe£ØNO3£©3ČÜŅŗ£® | ČÜŅŗ±äĪŖŗģÉ«£® |
| ²½Öč3£ŗĻņ²½Öč2µÄČÜŅŗÖŠ£¬¼ĢŠų¼ÓČė5µĪ 3mol•L-1AgNO3ČÜŅŗ£® | ĻÖĻóa³öĻÖ°×É«³Įµķ£¬ČÜŅŗŗģÉ«±äĒ³£® |
| ²½Öč4£ŗĻņ²½Öč1ÓąĻĀµÄ×ĒŅŗÖŠ¼ÓČė5µĪ 3mol•L-1KIČÜŅŗ£® | ³öĻÖ»ĘÉ«³Įµķ£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪļÖŹ | N2 | H2 | NH3 |
| ĘšŹ¼£Ømol£© | 2 | 7 | 0 |
| 10s£Ømol£© | 1.6 | ||
| Ę½ŗā£Ømol£© | 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijŠ£æŖÕ¹æĪĶāŃŠ¾æŠŌѧĻ°£ŗ“Ó·Ļ¾Éøɵē³ŲÖŠ»ŲŹÕĢ¼°ō”¢MnO2”¢NH4Cl”¢ZnCl2µČĪļÖŹ£¬Õūøö¹ż³ĢČēĻĀ£¬»Ų“šÓŠ¹ŲĪŹĢā£ŗ
ijŠ£æŖÕ¹æĪĶāŃŠ¾æŠŌѧĻ°£ŗ“Ó·Ļ¾Éøɵē³ŲÖŠ»ŲŹÕĢ¼°ō”¢MnO2”¢NH4Cl”¢ZnCl2µČĪļÖŹ£¬Õūøö¹ż³ĢČēĻĀ£¬»Ų“šÓŠ¹ŲĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
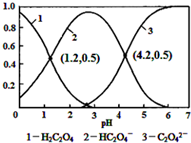
| A£® | pH=1.2ČÜŅŗÖŠ£ŗc£ØK+£©+c£ØH+£©ØTc£ØOH-£©+c£ØH2C2O4£© | |
| B£® | pH=2.7ČÜŅŗÖŠ£ŗ$\frac{{c}^{2}£ØH{C}_{2}{O}_{4}^{-}£©}{c£Ø{H}_{2}{C}_{2}{O}_{4}£©}$”Įc£ØC2O42-£©=1000 | |
| C£® | ½«ĻąĶ¬ĪļÖŹµÄĮæKHC2O4ŗĶK2C2O4¹ĢĢåĶźČ«ČÜÓŚĖ®ĖłµĆ»ģŗĻŅŗµÄpHĪŖ4.2 | |
| D£® | ĻņpH=1.2µÄČÜŅŗÖŠ¼ÓKOHČÜŅŗ½«pHŌö“óÖĮ4.2µÄ¹ż³ĢÖŠĖ®µÄµēĄė¶ČŅ»¶ØŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś | B£® | ¢Ś¢Ū | C£® | ¢Ū¢Ü | D£® | ¢Ł¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
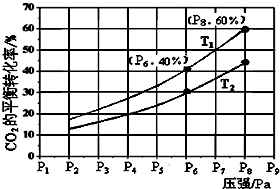
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com