
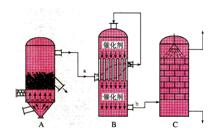
A����������ĽӴ������ڽӴ����з��� | B����������Ũ��Ϊ98.3%Ũ���������������� |
| C�����պ���48%�Ļ�����ʱ����FeS2��ʧ��2%����S��ʧ2% | |
| D��Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� |
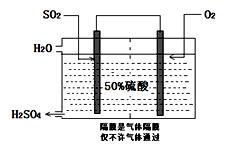
 �������CO2��Դ�� ��
�������CO2��Դ�� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
  ѹǿ ѹǿSO2�� ת���� �¶� | 1��105 Pa | 5��105 Pa | 10��105 Pa | 50��105 Pa | 100��105 Pa |
| 450 �� | 97.5% | 98.9% | 99.2% | 99.6% | 99.7% |
| 550 �� | 85.6% | 92.9% | 94.9% | 97.7% | 98.3% |
 �Ŀ�����Ϊ��________��
�Ŀ�����Ϊ��________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
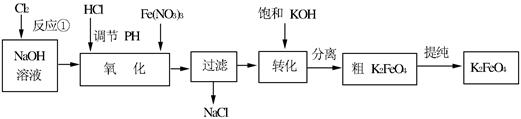
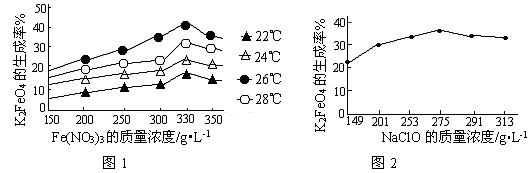
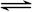 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���| A��H2O | B��CH3COONa������� | C��NH4Cl������� | D��Fe(NO3)3������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ���̿��Ʊ�������ص�һ�ֹ������̡�
���̿��Ʊ�������ص�һ�ֹ������̡�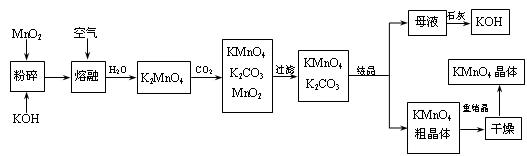
| A��75%�ƾ� | B��˫��ˮ | C������ | D��84����Һ��NaClO��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ��Һ�Ⱦ�ˮ��ԭ����ͬ |
| B���Ʋ������ͭ����ȶ�п�������ʴ |
| C����Ҫʱ����в����⻯�������ɴﵽ�˹������Ŀ�� |
| D��̼���ƿ����ڱ��Ƹ�㣬Ҳ����������θ�����֢ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
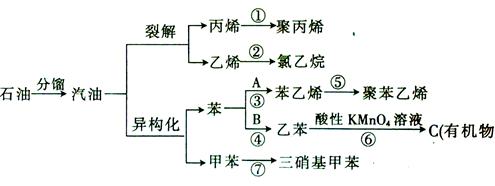
 ____________________________________________��
____________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���⣨������ | B���壨��ˮɹ�κ����Һ�� |
| C����ϩ���Ҵ��� | D��˳����ʯ���ѽ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
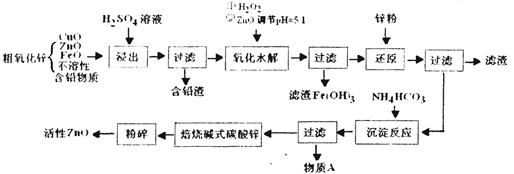
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú���͵ķ��� | B��ú�ĸ��� | C��ú������ | D��ú��Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com