
| A���Ȼ�����Һ�м���ͭ�ۣ�Fe3++Cu�TFe2++Cu2+ |
| B�����������ʵ�����NaHCO3��Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-�TBaCO3��+H2O |
| C������SO2ͨ����������Һ�У�Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO |
| D������AlCl3��Һ���������ˮ�У�Al3++4NH3?H2O�TAlO2-+4NH4++2H2O |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡĵ����һ��2010��2011ѧ��߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�058
(1)2003���ģ�ȫ��ѧ��ͶƱ��ѡ�˻�ѧʷ��ʮ��������ʵ�飬1848���˹�����ֹ��ڹ�ѧ�����°����;�ʯ���ξ�������;�ʯ���ξ���ֿ���ʵ�鱻ѡΪʮ��֮�ף���ش��������⣺
a�����;�ʯ���ξ�������;�ʯ���ξ���Ĺ�ϵ��________��
b�����л������о����������;�ʯ���εĹ�ϵ��ͬ����________��
(����ű�ʾ)
�ٱ�ϩ�����
��������
��CHClBrCH2F2
�ܸ���
��2����������
������
(2)��ѧ��һ��������ѧ�ƣ���������չʾ���������з���ԭ�ӵĹ��ɣ�����������������Թ��ɣ������������������ᰴ��������������һ�飬������________��________��________��________��(����ű�ʾ)
��H3PO4
��HClO
��H3BO3
��HNO2
(3)����Ԫ�ؿ�ν�Dz����Ի����������۵���ߵ��٣��й��ؽ�����ijԪ�صĻ�̬ԭ�ӵ����������ӵ�3d�ܼ�Ϊ�����״̬����������ӳ����Լ���________����Ӧ�����ӷ���ʽΪ________��������������Χ�����Ų�ͼ��________���ѱ���Ϊδ���������ѵĻ�����TiCl3��6H2O(��Է�������Ϊ262.5)��λ��Ϊ6��ȡ�þ���26.25�������Һ������������������Һ�����ˣ�ϴ�ӣ���ɣ����أ�����Ϊ28.70�ˣ���þ���Ļ�ѧʽӦ��ʾΪ________��
(4)���龧�徧���ṹ��ͼ����һ��������ӽ������ڵļ��������________����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
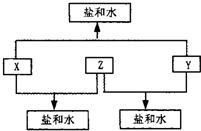
ij��������X�������й�ϵͼ������A��B�ֱ���X�������ۡ������۽������ӣ���ش�

��1��д��X������ ��Y�Ļ�ѧʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
�� X��ϡ���ᷴӦ����A��ij����������ӷ���ʽ
�� +2�۵�A��Y������Ӧ����+3�۵�B�����ӷ���ʽ
�� X��ĩ������ͭ��Һ�����û���Ӧ�����ӷ���ʽ
�� X�������������X2O3�������۷�Ӧ�Ļ�ѧ����ʽ
��3��A��Һ��NaOH��Һ�ڿ����з�Ӧ������ ��
��д���йصĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ����������к����ؽ����Ҹ߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
ij��������X�������й�ϵͼ������A��B�ֱ���X�������ۡ������۽������ӣ���ش�
��1��д��X������ ��Y�Ļ�ѧ ʽ ��
ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
�� X��ϡ���ᷴӦ����A��ij����������ӷ���ʽ
�� +2�۵�A��Y������Ӧ����+3�۵�B�����ӷ���ʽ
�� X��ĩ������ͭ��Һ�����û���Ӧ�����ӷ���ʽ

�� X�������������X2O3�������۷�Ӧ�Ļ�ѧ����ʽ
��3��A��Һ��NaOH��Һ�ڿ����з�Ӧ������  ��
��
��д���йصĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ���բ����������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����仯�������������������й㷺Ӧ�á���ش��������⣺
��1��ˮ�ȷ��Ʊ�Fe3O4�������ķ�Ӧ�ǣ�3Fe2����2S2O32-��O2��xOH����Fe3O4����S4O32-��2H2O
���������ӷ���ʽ��x��________��
��ÿ����1 mol Fe3O4����Ӧת�Ƶĵ���Ϊ________mol��
��2��ij��Ч��ˮ������Fe��OH��SO4�ۺϵõ�����ҵ����FeSO4��NaNO2��ϡ����Ϊԭ�����Ʊ�Fe��OH��SO4����Ӧ����NO���ɣ���ѧ����ʽΪ����������������
��¯���������з�������Ҫ��ӦΪ�� Fe2O3��s��+ CO��g��
Fe2O3��s��+ CO��g��

 Fe��s��+CO2��g��+Q
Fe��s��+CO2��g��+Q
��֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
|
�¶�/�� |
1000 |
1115 |
1300 |
|
ƽ�ⳣ�� |
4.0 |
3.7 |
3.5 |
��3���÷�Ӧ��ƽ�ⳣ������ʽK=_ ��Q 0���>������<����=������
��4�������������Ӧ��CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
A����߷�Ӧ�¶� B���Ƴ�����CO2
C��������ʵĴ��� D����С�������ݻ�
��5����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0 mol����ʱv�� v��������ڡ��������ڡ���С�ڡ���������l0 min����1000��ﵽƽ�⣬���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ����v ��CO2��= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com