
��ͼK381�ǡ�MnO2��H2O2�ֽⷴӦ����Ӱ����о���������ʾ��ͼ�������й�˵������ȷ����(����)
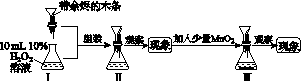
ͼK381
A��ʵ��ʱ�ȼ�H2O2��Һ���MnO2������������̡�Һ�ĽӴ����
B��Ϊʹʵ��˳�����У�H2O2��Һ���˴�ͼ��©��������
C�����ɹ۲쵽Ѹ�ٲ����������ݣ��������ľ����ȼ
D�����������в����ȼ���MnO2���ٲ���������ľ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�ѧ����ʽ��2N2O 2N2 + O2 ����Ӧ��ʼʱ��c(O2) ��c(N2)��Ϊ0��
2N2 + O2 ����Ӧ��ʼʱ��c(O2) ��c(N2)��Ϊ0��
c(N2O)=c mol��L��1��t min��c(O2)=a mol��L��1����
��1�� t minʱ c(N2)Ϊ ��
��2�� t minʱ c(N2O)Ϊ ��
��3�� t min�� O2ƽ����Ӧ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
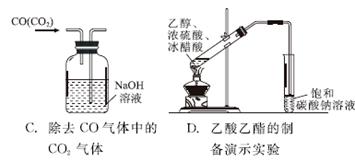
����ͼʾʵ����ȷ����(����)
 ��
��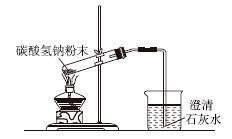
A.��ȥ������Һ,�еIJ���� B��̼���������ȷֽ�
��
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��.���������ϡ�
(1)Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡ���ᡣ
��.���Ʊ���Ʒ��
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
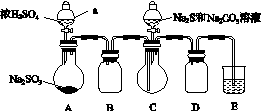
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�Ļ����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�еĻ�����Һ��________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.��̽���뷴˼��
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡ���ᡢ����ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________________________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����______________________________________________________________________________��
(3)Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��________�����ᴿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС����CoCl2��6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�� ��
�ٰ��IJⶨ����ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL cl mol��L��1���������Һ���ա�����������ȡ�½���ƿ����c2 mol��L��1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ��

���IJⶨװ��(��ʡ�Լ��Ⱥͼг�װ��)
���ȵIJⶨ��ȷ��ȡ��ƷX�������Һ����AgNO3����Һ�ζ���K2CrO4��ҺΪָʾ���������ֵ���ɫ����������ʧΪ�յ�(Ag2CrO4Ϊש��ɫ)��
�ش��������⣺
(1)װ���а�ȫ�ܵ�����ԭ����__________________________________________��
(2)��NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ��________ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ________��
(3)��Ʒ�а���������������ʽΪ________��
(4)�ⶨ��ǰӦ�ö�װ�ý��������Լ��飬�������Բ��òⶨ�����________(�ƫ�ߡ���ƫ�͡�)��
(5)�ⶨ�ȵĹ����У�ʹ����ɫ�ζ��ܵ�ԭ����____________________���ζ��յ�ʱ������Һ��c(Ag��)��2.0��10��5 mol��L��1��c(CrO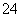 )Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
)Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
(6)���ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3���ܵĻ��ϼ�Ϊ________���Ʊ�X�Ļ�ѧ����ʽΪ______________________________________��X���Ʊ��������¶Ȳ��ܹ��ߵ�ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϊ�������ݿ��ŵ��� ( )
A. ij��ˮ��pHΪ5��6
B��ij����ʯ��ˮ��Ũ����2��0 mol/l
C��ij�������ӵ�ֱ����160 nm
D��ij����������ܶ�Ϊ1��8 g��/cm3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� ( )
A��һ����������1 L������ͨ��46 g NO2����NO2�����ʵ���Ũ��һ��Ϊ1 mol��l
B����״���£�22��4 l�ļ�������20X6��02 X 1023��ԭ��
C��1 mol��������ˮת��6��02X 1023��e��
D��0��1 mol CH5+��6��02 X lO23��eһ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ը��������Һ�����һ�������� �� ��
A����ϩ ��Ȳ B���� ���� C������ ������ D���� �ױ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮż����Ӧ��һ�����͵�ֱ���������Ӧ�����磺
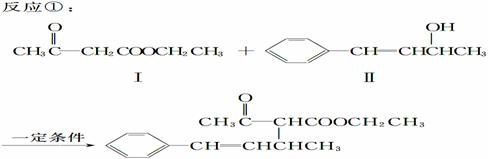
(1)���������ķ���ʽΪ________��
1 mol��������ȫȼ��������Ҫ����________ mol O2��
(2)����������ʹ________��Һ(��дһ��)��ɫ��
�������(����ʽΪC10H11Cl)����NaOHˮ��Һ�������ɻ������
��Ӧ�Ļ�ѧ����ʽΪ_____ ____��
(3)�����������NaOH�Ҵ���Һ�������ɻ�����������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2�����Ľṹ��ʽΪ__________��
(4)����CH3COOCH2CH3�ɺϳɻ�������������CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ��������
���Ľṹ��ʽΪ__________�����Ľṹ��ʽΪ______________��
(5)��һ�������£� ��
�� 
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com