
ŹŅĪĀĻĀ£¬ŌŚ25 mL 0.1 mol£ÆLNaOHČÜŅŗÖŠÖšµĪ¼ÓČė0.2 mol£ÆL CH3COOHČÜŅŗ£¬ĒśĻßČēÓŅĶ¼ĖłŹ¾£¬ČōŗöĀŌĮ½ČÜŅŗ»ģŗĻŹ±µÄĢå»ż±ä»Æ£¬ÓŠ¹ŲĮ£×ÓÅØ¶Č¹ŲĻµ±Č½Ļ“ķĪóµÄŹĒ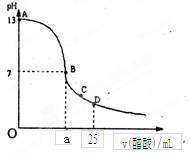
A£®ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠŅ»¶Ø¶¼ÓŠ£ŗc(Na+)+c(H+)= c(CH3COO£)+c(OH£)
B£®ŌŚBµć£ŗa>12.5£¬ĒŅÓŠc(Na+)=c(CH3COO£)=c(OH£)=c(H+)
C£®ŌŚCµć£ŗc(Na+)>c(CH3COO£) >c(H+)>c(OH£)
D£®ŌŚDµć£ŗc(CH3COO£)+c(CH3COOH)£½0.1mol/L
BC
½āĪöæ¼µć£ŗĖį¼ī»ģŗĻŹ±µÄ¶ØŠŌÅŠ¶Ļ¼°ÓŠ¹ŲphµÄ¼ĘĖć£»Ąė×ÓÅØ¶Č“óŠ”µÄ±Č½Ļ£®
·ÖĪö£ŗŌŚ25mL 0.1mol£®L-1NaOHČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol?L-1 CH3COOH ČÜŅŗ£¬¶žÕßÖ®¼äĻą»„·“Ó¦£¬µ±Ē”ŗĆĶźČ«·“Ó¦Ź±£¬ĖłŠč“×ĖįµÄĢå»żĪŖ12.5mL£¬µ±·“Ó¦ÖĮČÜŅŗĻŌÖŠŠŌŹ±£¬“×ĖįÓ¦ÉŌ¹żĮ棬ĒŅc£ØOH-£©=c£ØH+£©£¬×¢Ņāøł¾ŻµēŗÉŹŲŗćĖ¼ĻėĄ“±Č½ĻĄė×ÓÅØ¶Č“óŠ”£®
½ā£ŗA”¢ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠÖ»“ęŌŚĖÄÖÖĄė×ÓÓŠNa+”¢H+”¢CH3COO-”¢OH-£¬øł¾ŻµēŗÉŹŲŗćŌņÓŠ£ŗ
c£ØNa+£©+c£ØH+£©=c£ØCH3COO-£©+c£ØOH-£©£¬¹ŹAÕżČ·£»
B”¢ŌŚBµćČÜŅŗĻŌÖŠŠŌ£¬Ōņ½į¹ūŹĒc£ØOH-£©=c£ØH+£©£¬øł¾ŻµēŗÉŹŲŗćc£ØNa+£©+c£ØH+£©=c£ØCH3COO-£©+c£ØOH-£©£¬ŌņŅ»¶ØÓŠ
c£ØNa+£©=c£ØCH3COO-£©£¬ČÜŅŗµÄ³É·ÖĪŖ£ŗ·“Ӧɜ³ÉµÄ“×ĖįÄĘŗĶŹ£ÓąµÄ“×Ėį£¬“×ĖįÄʵÄĖ®½ā³Ģ¶ČŗĶ“×ĖįµÄµēĄė³Ģ¶ČĻąµČ£¬¹ŹÓŠ£ŗc£ØNa+£©=c£ØCH3COO-£©£¾c£ØOH-£©=c£ØH+£©£¬¹ŹB“ķĪó£»
C”¢ŌŚCµć£¬ČÜŅŗĻŌĖįŠŌ£¬¹ŹÓŠc£ØOH-£©£¼c£ØH+£©£¬øł¾ŻµēŗÉŹŲŗć£ŗc£ØNa+£©+c£ØH+£©=c£ØCH3COO-£©+c£ØOH-£©£¬¹Źc£ØNa+£©£¼c£ØCH3COO-£©£¬¹ŹC“ķĪó£»
D”¢ŌŚDµćŹ±£¬“×ĖįŹ£Óą£¬Ź£ÓąµÄ“×ĖįµÄÅضČŗĶÉś³ÉµÄ“×ĖįÄĘÅضČĻąµČ¾łĪŖ0.05mol/l£¬øł¾ŻĪļĮĻŹŲŗć£¬Ōņ£ŗ
c£ØCH3COO-£©+c£ØCH3COOH£©=0.1mol?L-1£¬¹ŹDÕżČ·£»
¹ŹŃ”BC£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¹ć¶«Ź”ÉĒĶ·ŹŠ½šÉ½ÖŠŃ§2011£2012ѧğø߶žÉĻѧʌ12ŌĀŌĀæ¼»ÆѧŹŌĢā ĢāŠĶ£ŗ021
|
ŹŅĪĀĻĀ£¬ ŌŚ25 mL””0.1 mol/L””NaOHČÜŅŗÖŠÖšµĪ¼ÓČė0.2 mol/L””CH3COOHČÜŅŗ£¬ĒśĻßČēĻĀĶ¼ĖłŹ¾£¬ČōŗöĀŌĮ½ČÜŅŗ»ģŗĻŹ±µÄĢå»ż±ä»Æ£¬ÓŠ¹ŲĮ£×ÓÅØ¶Č¹ŲĻµ±Č½Ļ“ķĪóµÄŹĒ
| |
A£® |
ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠŅ»¶Ø¶¼ÓŠ£ŗc(Na+)£«c(H+)£½c(CH3COO£)£«c(OH£) |
B£® |
ŌŚBµć£ŗa£¾12.5£¬ĒŅÓŠc(Na+)£½c(CH3COO£)£½c(OH£)£½c(H+) |
C£® |
ŌŚCµć£ŗc(Na+)£¾c(CH3COO£)£¾c(H+)£¾c(OH£) |
D£® |
ŌŚDµć£ŗc(CH3COO£)£«c(CH3COOH)£½0.1 mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ¹ć¶«Ź”ÉĒĶ·ŹŠø߶ž12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
ŹŅĪĀĻĀ£¬ŌŚ25 mL 0.1 mol£ÆLNaOHČÜŅŗÖŠÖšµĪ¼ÓČė0.2 mol£ÆL CH3COOHČÜŅŗ£¬ĒśĻßČēÓŅĶ¼ĖłŹ¾£¬ČōŗöĀŌĮ½ČÜŅŗ»ģŗĻŹ±µÄĢå»ż±ä»Æ£¬ÓŠ¹ŲĮ£×ÓÅØ¶Č¹ŲĻµ±Č½Ļ“ķĪóµÄŹĒ
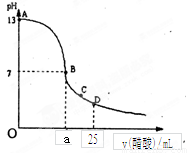
A£®ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠŅ»¶Ø¶¼ÓŠ£ŗc(Na+)+c(H+)= c(CH3COO£)+c(OH£)
B£®ŌŚBµć£ŗa>12.5£¬ĒŅÓŠc(Na+)=c(CH3COO£)=c(OH£)=c(H+)
C£®ŌŚCµć£ŗc(Na+)>c(CH3COO£) >c(H+)>c(OH£)
D£®ŌŚDµć£ŗc(CH3COO£)+c(CH3COOH)£½0.1mol/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(08»ŻÖŻµ÷ŃŠ)ŹŅĪĀĻĀ£¬ŌŚ25 mL 0.1 mol£ÆLNaOHČÜŅŗÖŠÖšµĪ¼ÓČė0.2 mol£ÆL CH3COOHČÜŅŗ£¬
ĒśĻßČēĻĀĶ¼ĖłŹ¾£¬ČōŗöĀŌĮ½ČÜŅŗ»ģŗĻŹ±µÄĢå»ż±ä»Æ£¬ÓŠ¹ŲĮ£×ÓÅØ¶Č¹ŲĻµ±Č½ĻÕżČ·µÄŹĒ£Ø£©
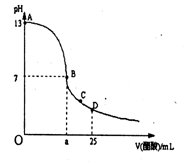 A.ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠŅ»¶Ø¶¼ÓŠ c(Na+)+ c(H+)= c(CH3COO£)+c(OH£)
A.ŌŚA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠŅ»¶Ø¶¼ÓŠ c(Na+)+ c(H+)= c(CH3COO£)+c(OH£)
B.ŌŚBµć£ŗa=12.5£¬
C.ŌŚCµć£ŗc(CH3COO£) > c(Na+) >c(H+)>c(OH£)
D.ŌŚDµć£ŗc(CH3COO£) =c(CH3COOH)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com