
ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų¹¤×÷Ź±µÄŹ¾ŅāĶ¼£¬ŅŅ³ŲÖŠµÄĮ½øöµē¼«Ņ»øöŹĒŹÆÄ«µē¼«£¬Ņ»øöŹĒĢśµē¼«£¬¹¤×÷Ź±M”¢NĮ½øöµē¼«µÄÖŹĮ涼²»¼õÉŁ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©Mµē¼«µÄ²ÄĮĻŹĒ £¬µē¼«Ćū³ĘŹĒ £¬¼ÓČėŅŅ“¼µÄ²¬µē¼«µÄµē¼«·“Ó¦
Ź½ĪŖ ”£Š“³öŅŅ³ŲÖŠ·¢ÉśµÄ»Æѧ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø2£©ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³Ų֊ijŅ»µē¼«Īö³ö½šŹōŅų4£®32gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄŃõĘųĪŖ L£Ø±ź×¼×“æöĻĀ£©£»Čō“ĖŹ±ŅŅ³ŲČÜŅŗµÄĢå»żĪŖ400mL£¬ŌņŅŅ³ŲÖŠČÜŅŗµÄpHĪŖ ”£
£Ø3£©ČōŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö29£®71kJČČĮ攣±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©¼×ĶéŅ²ŹĒŅ»ÖÖŗÜŗƵÄĒå½ąÄÜŌ“”£ŌĢ²ŲŌŚŗ£µ×µÄ”°æÉČ¼±ł”±ŹĒøßŃ¹ĻĀŠĪ³ÉµÄĶā¹ŪĻń±łµÄ¼×ĶéĖ®ŗĻĪļ¹ĢĢ唣¼×ĶéĘųĢåČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗCH4(g)£«2O2(g)£½CO2(g)£«2H2O(l) ”÷H£½£890£®3 kJ£Æmol”£356g”°æÉČ¼±ł”±(Čō·Ö×ÓŹ½ĪŖCH4”¤9H2O)ŹĶ·ÅµÄ¼×ĶéĘųĢåĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£®·Å³öµÄČČĮæĪŖ kJ”£
£Ø1£©Ģś
Ņõ¼« C2H5OH£12e+16OH”Ŗ=2CO32”Ŗ+11H2O
4Ag++2H2O 4Ag+4H++O2”ü£®
4Ag+4H++O2”ü£®
£Ø2£©0£®224 1 £Ø3£©C2H5OH£Øl£©+3O2£Øg£© 2CO2£Øg£©+3H2O£Øl£©”÷H=£1366£®7kJ/mol £Ø4£©1780£®6
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ÓÉĶ¼æÉ擳ö£ŗ¼×ŹĒČ¼ĮĻµē³Ų”£ŹōÓŚŌµē³Ų”£ŅŅŌņŹĒµē½ā³Ų”£¶ŌÓŚ¼×³ŲĄ“Ėµ£ŗĶØČėŅŅ“¼µÄµē¼«Ź½øŗ¼«£¬ĶØČėŃõĘųµÄµē¼«ŹĒÕż¼«”£¶ŌÓŚŅŅ³ŲĄ“Ėµ£ŗMŹĒŅõ¼«£¬NŹĒŃō¼«”£ŅŅ³ŲÖŠµÄĮ½øöµē¼«Ņ»øöŹĒŹÆÄ«µē¼«£¬Ņ»øöŹĒĢśµē¼«£¬¹¤×÷Ź±M”¢NĮ½øöµē¼«µÄÖŹĮ涼²»¼õÉŁ£¬ĖµĆ÷Ģśµē¼«ŹĒŅõ¼«£ØM¼«£©ŹÆÄ«µē¼«ŹĒŃō¼«£ØN¼«£©”£ŅņĪŖČē¹ūĢśµē¼«×÷Ńō¼«£¬¾Ķ»į·¢ÉśŃõ»Æ·“Ó¦µ¼ÖĀÖŹĮæ¼õÉŁ”£¼ÓČėŅŅ“¼µÄ²¬µē¼«µÄµē¼«·“Ó¦£ŗC2H5OH£12e+16OH”Ŗ=2CO32”Ŗ+11H2O”£ŅŅ³Ų¾ĶŹĒµē½āĻõĖįŅųČÜŅŗ”£·¢ÉśµÄ»Æѧ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ4Ag++2H2O 4Ag+4H++O2”ü£®£Ø2£©ŌŚÕūøö±ÕŗĻ»ŲĀ·ÖŠµē×Ó×ŖŅĘŹżÄæĻąµČ”£n(Ag)=m/M=4£®32g”Ā108g/mol=0£®04mol£®n(e-)=0£®04mol£®ĖłŅŌn(O2)=0£®04mol”Ā4=0£®01mol£®yŅņĪŖn=V/VM,ĖłŅŌV=n”¤VM=0£®01mol”Į22£®4mol/L=0£®224L£®ŅņĪŖn(Ag)=n(H+)=0£®04mol£®V(aq)=0£®4LĖłŅŌC(H+)=n/V£Øaq£©=0£®04mol”Ā0£®4L=0£®1mol/L£®¹ŹPH=1£Ø3£©ŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ46£¬ČōŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö29£®71kJČČĮ攣46gŅŅ“¼1molČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³öČČĮæ46”Į29£®71kJ=1366£®7kJ£®±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC2H5OH£Øl£©+3O2£Øg£©
4Ag+4H++O2”ü£®£Ø2£©ŌŚÕūøö±ÕŗĻ»ŲĀ·ÖŠµē×Ó×ŖŅĘŹżÄæĻąµČ”£n(Ag)=m/M=4£®32g”Ā108g/mol=0£®04mol£®n(e-)=0£®04mol£®ĖłŅŌn(O2)=0£®04mol”Ā4=0£®01mol£®yŅņĪŖn=V/VM,ĖłŅŌV=n”¤VM=0£®01mol”Į22£®4mol/L=0£®224L£®ŅņĪŖn(Ag)=n(H+)=0£®04mol£®V(aq)=0£®4LĖłŅŌC(H+)=n/V£Øaq£©=0£®04mol”Ā0£®4L=0£®1mol/L£®¹ŹPH=1£Ø3£©ŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ46£¬ČōŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö29£®71kJČČĮ攣46gŅŅ“¼1molČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³öČČĮæ46”Į29£®71kJ=1366£®7kJ£®±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC2H5OH£Øl£©+3O2£Øg£© 2CO2£Øg£©+3H2O£Øl£©”÷H=£1366£®7kJ/mol £Ø4£©æÉČ¼±łĻą¶Ō·Ö×ÓÖŹĮæĪŖ178£¬n(æÉČ¼±ł)=356g”Ā108g/mol=2mol£®£®ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£®·Å³öµÄČČĮæĪŖ2mol”Į890£®3 kJ£Æmol=1780£®6KJ
æ¼²éŌµē³Ų”¢µē½ā³Ų¼°ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“µČÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų¹¤×÷Ź±µÄŹ¾ŅāĶ¼£¬ŅŅ³ŲÖŠµÄĮ½øöµē¼«Ņ»øöŹĒŹÆÄ«µē¼«£¬Ņ»øöŹĒĢśµē¼«£¬¹¤×÷Ź±M”¢NĮ½øöµē¼«µÄÖŹĮ涼²»¼õÉŁ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
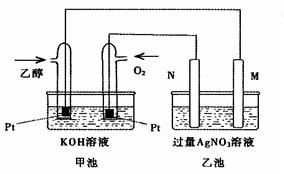
£Ø1£©Mµē¼«µÄ²ÄĮĻŹĒ £¬µē¼«Ćū³ĘŹĒ £¬NµÄµē¼«·“Ó¦Ź½ĪŖ £¬¼ÓČėŅŅ“¼µÄ²¬µē¼«µÄµē¼«·“Ó¦Ź½ ”£
£Ø2£©ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³Ų֊ijŅ»µē¼«Īö³ö½šŹōŅų4.32gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄŃõĘųĪŖ L£Ø±ź×¼×“æöĻĀ£©£»Čō“ĖŹ±ŅŅ³ŲČÜŅŗµÄĢå»żĪŖ400mL£¬ŌņŅŅ³ŲÖŠČÜŅŗµÄpHĪŖ ”£
£Ø3£©ČōŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö29.71kJČČĮ棬±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010”Ŗ2011ѧğÕć½Ź”ŗ¼ÖŻŹ¦·¶“óѧø½Źō֊ѧø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©Óė¼×“¼Č¼ĮĻµē³ŲĻą±Č£¬ŅŅ“¼Č¼ĮĻµē³Ų¾ßÓŠ¶¾ŠŌµĶ”¢ĄķĀŪÄÜĮæĆܶČøßµČÓÅµć£¬Ņņ“Ė±»¹ć·ŗČĻĪŖŹĒøüÓŠĒ°Ķ¾µÄČ¼ĮĻµē³Ų”£ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų¹¤×÷Ź±µÄŹ¾ŅāĶ¼”£ŅŅ³ŲÖŠµÄĮ½øöµē¼«¾łĪŖŹÆÄ«µē¼«£¬ŅŅ³ŲÖŠŹ¢ÓŠ100 mL3.00 mol/LµÄCuSO4ČÜŅŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ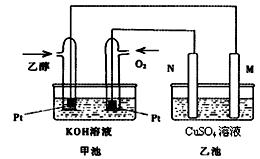
(1) ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö30kJČČĮ棬±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
(2)NµÄµē¼«·“Ó¦Ź½ĪŖ ”£
(3)ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³Ų֊ijŅ»µē¼«Īö³ö½šŹōĶ6.4gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄŃõĘų Éż
£Ø±ź×¼×“æöĻĀ£©
(4) ŌŚ“Ė¹ż³ĢÖŠ£¬ČōŅŅ³ŲÖŠĮ½µē¼«²śÉśµÄĘųĢåĒ”ŗĆĻąµČŹ±(¼ŁÉč±ź×¼×“æö ĻĀ)£¬ĄķĀŪÉĻŠčĶØČėŅŅ“¼ g£æ
ĻĀ)£¬ĄķĀŪÉĻŠčĶØČėŅŅ“¼ g£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģÕć½Ź”ŗ¼Ź®ĖÄÖŠøßČżÉĻѧʌ11ŌĀŌĀæ¼»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų¹¤×÷Ź±µÄŹ¾ŅāĶ¼£¬ŅŅ³ŲÖŠµÄĮ½øöµē¼«Ņ»øöŹĒŹÆÄ«µē¼«£¬Ņ»øöŹĒĢśµē¼«£¬¹¤×÷Ź±M”¢NĮ½øöµē¼«µÄÖŹĮ涼²»¼õÉŁ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Mµē¼«µÄ²ÄĮĻŹĒ £¬µē¼«Ćū³ĘŹĒ £¬NµÄµē¼«·“Ó¦Ź½ĪŖ £¬¼ÓČėŅŅ“¼µÄ²¬µē¼«µÄµē¼«·“Ó¦Ź½ ”£
£Ø2£©ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³Ų֊ijŅ»µē¼«Īö³ö½šŹōŅų4.32gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄŃõĘųĪŖ L£Ø±ź×¼×“æöĻĀ£©£»Čō“ĖŹ±ŅŅ³ŲČÜŅŗµÄĢå»żĪŖ400mL£¬ŌņŅŅ³ŲÖŠČÜŅŗµÄpHĪŖ ”£
£Ø3£©ČōŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö29.71kJČČĮ棬±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŗŚĮś½Ź”øßČżÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų³£ĪĀ¹¤×÷ŌĄķŹ¾ŅāĶ¼£¬ŅŅ³ŲÖŠµÄĮ½øöµē¼«Ņ»øö
ŹĒŹÆÄ«µē¼«£¬Ņ»øöŹĒĢśµē¼«”£¹¤×÷Ź±M”¢NĮ½øöµē¼«µÄÖŹĮ涼²»¼õÉŁ£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
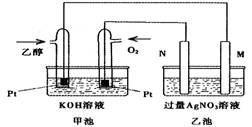 A.Mµē¼«µÄ²ÄĮĻŹĒŹÆÄ«
A.Mµē¼«µÄ²ÄĮĻŹĒŹÆÄ«
B.ČōŅŅ³Ų֊ijŅ»µē¼«ÖŹĮæŌö¼Ó4.32gŹ±£¬ĄķĀŪÉĻĻūŗÄŃõĘųĪŖ448ml
C.ŌŚ“Ė¹ż³ĢÖŠ£¬¼×³ŲÖŠOH-ĻņĶØŅŅ“¼µÄŅ»¼«ŅʶÆ
D.ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³ŲČÜŅŗÖŠµē×Ó“ÓMµē¼«ĻņNµē¼«ŅʶÆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÕć½Ź”ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©Óė¼×“¼Č¼ĮĻµē³ŲĻą±Č£¬ŅŅ“¼Č¼ĮĻµē³Ų¾ßÓŠ¶¾ŠŌµĶ”¢ĄķĀŪÄÜĮæĆܶČøßµČÓÅµć£¬Ņņ“Ė±»¹ć·ŗČĻĪŖŹĒøüÓŠĒ°Ķ¾µÄČ¼ĮĻµē³Ų”£ĻĀĶ¼ŹĒŅ»øöŅŅ“¼Č¼ĮĻµē³Ų¹¤×÷Ź±µÄŹ¾ŅāĶ¼”£ŅŅ³ŲÖŠµÄĮ½øöµē¼«¾łĪŖŹÆÄ«µē¼«£¬ŅŅ³ŲÖŠŹ¢ÓŠ100 mL3.00 mol/LµÄCuSO4ČÜŅŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
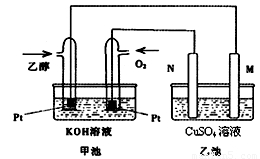
(1) ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬1g C2H5OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2OŹ±·Å³ö30kJČČĮ棬±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
(2)NµÄµē¼«·“Ó¦Ź½ĪŖ ”£
(3)ŌŚ“Ė¹ż³ĢÖŠ£¬ŅŅ³Ų֊ijŅ»µē¼«Īö³ö½šŹōĶ6.4gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄŃõĘų Éż
£Ø±ź×¼×“æöĻĀ£©
(4) ŌŚ“Ė¹ż³ĢÖŠ£¬ČōŅŅ³ŲÖŠĮ½µē¼«²śÉśµÄĘųĢåĒ”ŗĆĻąµČŹ±(¼ŁÉč±ź×¼×“æöĻĀ)£¬ĄķĀŪÉĻŠčĶØČėŅŅ“¼ g£æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com