
���� ��һ�ݼ�����NaHCO3 ��Һ�����Ⱥ��ռ�������0.03mol��������HCO3-+H+=CO2��+H2O����һ������H+����n��H+��=0.03mol������H+��CO32-���ܴ������棬��һ��������CO32-���ڶ��ݼ�����Ba��NO3��2 ��Һ����ַ�Ӧ����˸���ó���4.66g���ó���ΪBaSO4��˵����Һ��һ������SO42-����һ��������Ba2+����n��SO42-��=$\frac{4.66g}{233g/mol}$=0.02mol����֪��Һ��һ��������Ba2+��������Һ������ԭ��Ӧ������K+��Mg2+�е�����һ�֣��Դ˽��
��� �⣺��һ�ݼ�����NaHCO3 ��Һ�����Ⱥ��ռ�������0.03mol��������HCO3-+H+=CO2��+H2O����һ������H+����n��H+��=0.03mol������H+��CO32-���ܴ������棬��һ��������CO32-���ڶ��ݼ�����Ba��NO3��2 ��Һ����ַ�Ӧ����˸���ó���4.66g���ó���ΪBaSO4��˵����Һ��һ������SO42-����һ��������Ba2+����n��SO42-��=$\frac{4.66g}{233g/mol}$=0.02mol����֪��Һ��һ��������Ba2+��������Һ������ԭ��Ӧ������K+��Mg2+�е�����һ�֣��Դ˽�𣻣��ʴ�Ϊ����
��1���ɷ�����֪����Һ��һ�������ڵ������ǣ�Ba2+��CO32-���ʴ�Ϊ��Ba2+��CO32-��
��2��ԭ��Һ����ܲ����ڵ���K+��Mg2+ �е�һ�֣��ʴ�Ϊ��K+��Mg2+��
��3����ʵ��ڿ�֪����Һ��һ�����ڵ���������SO42-�������ʵ���Ũ��Ϊ$\frac{0.02mol}{0.1L}$=0.2 mol/L���ʴ�Ϊ��SO42-��0.2 mol/L��
���� ���⿼�����Ӽ��鼰�������ӹ������⣬�漰�й����ʵ��������ʵ���Ũ�ȼ��㣬��Ŀ�Ѷ��еȣ�����ע�������Һ������ԭ����ж���Һ��Ӧ����K+��Mg2+��


 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�е��ܽ��ԣ�NaHCO3��Na2CO3 | |
| B�� | ��ͬ�����£����Ũ�����ᷴӦ����CO2�����ʣ�NaHCO3��Na2CO3 | |
| C�� | ��������Na2CO3��NaHCO3�ֱ����������ᷴӦ��NaHCO3�ų�CO2���� | |
| D�� | �����ʵ�����Na2CO3��NaHCO3�ֱ����������ᷴӦ������CO2������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 35.5��108 | B�� | 16��207 | C�� | 8��1 | D�� | 108��35.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʹ��ǽ�̫����ת��Ϊ���ܵij��ò��� | |
| B�� | ���ø����������ҩ�ý��һ�����彡�����Σ�� | |
| C�� | ������������θ����кͼ� | |
| D�� | ����ˮ��ʱ�������������ԵĽ������ӣ�����Ư�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
����˵���У���ȷ����( )
A����ѧ��Ӧ������ָһ��ʱ�����κ�һ�ַ�Ӧ������ʵ����ļ��ٻ��κ�һ�����������ʵ���������
B����ѧ��Ӧ����Ϊ0.8mol��L��1��s��1��ָ1����ʱij���ʵ�Ũ��Ϊ0.8mol��L��1
C�����ݻ�ѧ��Ӧ���ʵĴ�С����֪����ѧ��Ӧ���еĿ���
D�������κλ�ѧ��Ӧ��˵����Ӧ����Խ�죬��Ӧ�����Խ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.05 mol/L | B�� | 0.01 mol/L | C�� | 0.02 mol/L | D�� | 0.03 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/L NH4Cl��Һ��0.05mol/L NaOH��Һ�������Ϻ����õļ�����Һ�У�c��Cl-����c��Na+����c��NH${\;}_{4}^{+}$����c��OH-����c��H+�� | |
| B�� | pH=2��HA��Һ��pH=12��MOH��Һ�������ϣ�c��M+��=c��A-����c��OH-��=c��H+�� | |
| C�� | �����ʵ�����NaClO��NaHCO5�����Һ�У�c��HClO��+c��ClO-���Tc��HCO3-��+c��H2CO3��+c��CO32-�� | |
| D�� | ij��Ԫ�������ʽ��NaHA��Һ��c��Na+��+c��H+��=c��OH-��+c��HA-��+c��A2-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��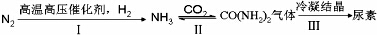
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com