
ij��Һ�п��ܺ���H����NH ��Mg2����Al3����Fe3����CO
��Mg2����Al3����Fe3����CO ��SO
��SO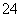 ��NO
��NO �еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���� (����)��
�еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���� (����)��

A����Һ�е�������ֻ��H����Mg2����Al3��
B����Һ��n(NH )��0.2 mol
)��0.2 mol
C����Һ��һ������CO �����ܺ���SO
�����ܺ���SO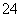 ��NO
��NO
D��n(H��)��n(Al3��)��n(Mg2��)��1��1��1
�������ɢ�֪��Һ��һ������H����һ��û��CO ��NO
��NO ���ɢ�֪��Һ��һ������Mg2����Al3������ͼ���֪��Һ�л�һ������NH
���ɢ�֪��Һ��һ������Mg2����Al3������ͼ���֪��Һ�л�һ������NH ����n(NH
����n(NH )��0.7 mol��0.5 mol��0.2 mol��n(H��)��0.1 mol��n(Al3��)��0.8 mol��0.7 mol��0.1 mol������Al3����Mg2��������0.4 mol OH�������г���Al3������0.3 mol OH��������Mg2������0.1 mol OH��������Mg2����2OH�����ɵ�n(Mg2��)��0.05 mol����ֻ��ѡ��B��ȷ��
)��0.7 mol��0.5 mol��0.2 mol��n(H��)��0.1 mol��n(Al3��)��0.8 mol��0.7 mol��0.1 mol������Al3����Mg2��������0.4 mol OH�������г���Al3������0.3 mol OH��������Mg2������0.1 mol OH��������Mg2����2OH�����ɵ�n(Mg2��)��0.05 mol����ֻ��ѡ��B��ȷ��
�𰸡�B


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ��ԭ����������ȷ���� (����)��
A��Ϊ��ֹ�ִ���ʴ�����ִ�����������Դ�ĸ�������
B����пƬ�϶�ͭ�����Ȼ�п��Һ�����Һ
C������Ȼ�����Һ�Ʊ�����������ʱ������������
D���õ�ⷨ����ͭʱ���������Ͽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��£���ӦA2(g)��B2(g)
 2AB(g)�ﵽƽ��ı�־��(����)
2AB(g)�ﵽƽ��ı�־��(����)
A����λʱ��������n mol��A2��ͬʱ����n mol��AB
B�������ڵ���ѹǿ����ʱ��ı仯���仯
C����λʱ��������2n mol��ABͬʱ����n mol��B2
D��ƽ����Է�����������ʱ��仯���仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɾ۱�ϩ����ά�ĵ����DZ�ϩ�棬���������������ַ����Ʊ���
����һ��CaCO3 CaO
CaO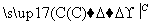 CaC2
CaC2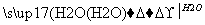 CH��CH
CH��CH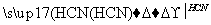 CH2===CH—CN
CH2===CH—CN
��������CH2===CH—CH3��NH3��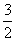 O2
O2 CH2===CH—CN��3H2O
CH2===CH—CN��3H2O
�ٷ������ȷ���һ��Ӧ�����٣���Դ���ĵͣ��ɱ��͡��ڷ������ȷ���һԭ�Ϸḻ�����ռ��۷������ȷ���һ�������ж������ʹ�ã���������Ⱦ���ܷ�������Ҫ��Ӧ�¶ȸߣ����ܴ�
�������������ַ���������ȷ����(����)
A���٢ڢ� B���٢ۢ�
C���ڢۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������ڳ�����һ���ܴ���������� (����)��
A��NaHS��Һ�У�SO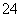 ��K����Cl����Cu2��
��K����Cl����Cu2��
B����c(H��)/c(OH��)��1012����Һ�У�NH ��NO
��NO ��K����Cl��
��K����Cl��
C�����������ܷų�H2����Һ�У�Mg2����NH ��NO
��NO ��Cl��
��Cl��
D��ͨ�����CO2����Һ�У�Na����ClO����CH3COO����HCO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijǿ������ҺX�п��ܺ���NH ��Fe2����Al3����CO
��Fe2����Al3����CO ��SO
��SO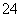 ��Cl����NO
��Cl����NO �е������֡�ij�о���ѧϰС��Ϊ̽����ҺX����ɣ���������ʵ��(����YΪ��ɫ��ζ������)��
�е������֡�ij�о���ѧϰС��Ϊ̽����ҺX����ɣ���������ʵ��(����YΪ��ɫ��ζ������)��

�ش��������⣺
(1)��ҺX�п϶����ڵ�������__________________���϶������ڵ�������____________________________________________________��
(2)д����������A�����ӷ���ʽ��____________________________________ _________________________________________________________________��
(3)д������ҺE��ͨ������Yһ�����������ӷ���ʽ��___________________ _________________________________________________________________��
(4)�������ʵ�鷽����һ��ȷ�����ܴ��ڵ����ӣ�д��ʵ��IJ������衢��������ۣ�_________________________________________________________ _________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������д������(����)
A���ù��Ϊ10 mL����Ͳ��ȡ6 mL��Һ��
B����ҩ����ֽ�۰ѷ�ĩ״ҩƷ�����Թܵĵײ�
C������ʱ��������ĩ��Ӧ�����������ֽ��
D�����û���ԹܼУ�������ʱ�ֳ��Թܸ������Һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧ���P������������ȷ����
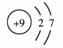 A����ϩ���ӵĽṹ��ʽ��CH2��CH2 B��
A����ϩ���ӵĽṹ��ʽ��CH2��CH2 B��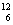 C��
C��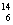 C��Ϊͬλ��
C��Ϊͬλ��
C�������ӽṹʾ��ͼ�� D����OH�ĵ���ʽ�� 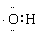
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com