
���ڹ�ҵ�� չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����
չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д����� SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
(1)д��������ҵ���������в���SO2��ʵ����
��________________________________________________________________________��
��________________________________________________________________________��
(2)����SO2��Ⱦ�ɲ��õĴ�ʩ��(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
��___________________ _____________________________________________________��
_____________________________________________________��
(3)ʪʽʯ��ʯ—ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������ Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ—ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�________________________________________________________________________
_____________ ___________________________________________________________��
___________________________________________________________��
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����__________________��
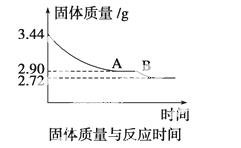
(4)ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4·xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ����ͼ��ʾ�����ݱ��������������Ϊ2.72 g���ٸı䡣��
��ʯ��Ļ�ѧʽ����ͼ����AB�ζ�Ӧ������Ļ�ѧʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.1.52gͭþ�Ͻ���ȫ�ܽ���50mL�ܶ�Ϊ1.40g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4 �Ļ������1120mL (��״��)����Ӧ�����Һ�м���1.0mol/LNaOH��Һ,����������ȫ������ʱ���õ�2.54g����������˵������ȷ���� �� ��
A.�úϽ���ͭ��þ�����ʵ���֮����2 ︰1
B.��Ũ������HNO3�����ʵ���Ũ����14.0mol/L
C. NO2��N2O4 �Ļ�������У�NO2 ���� ��������80%
��������80%
D. �õ�2.54����ʱ������NaOH��Һ�������600mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ط��š���Ӧ����ʽ���ṹʾ��ͼ������ʽ���ṹʽ��ͨ��������ѧ���
�����йػ�ѧ����ı�ʾ��������ȷ���ǣ� ��
A��H2O2�ĵ���ʽ��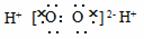
B��NH4I�ĵ���ʽ �� 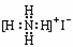
C��ԭ�Ӻ�����8�����ӵ�̼ԭ�ӣ�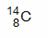
D��CO2���ӵĽṹʽ��O=C=O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ��
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��� ������ţ���
���û�������ȡ��ˮ ����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�� �ܸĽ��ء��塢þ����ȡ����
��2�����á���������������Ũ��ˮ�д���Br2�����ô������ա������������Ҫ��Ӧ��Br2+Na2CO3+H2O 
 NaBr + N
NaBr + N aBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ mol��
aBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ�����Ϊ mol��
��3����ˮ��þ��һ�ι�����������ͼ��
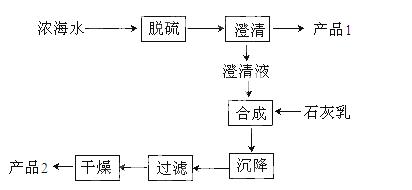
Ũ��ˮ����Ҫ�ɷ����£�
| ���� | N | Mg2+ | Cl- | SO42- |
| Ũ��/g/L | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ ����Ʒ2�Ļ�ѧʽΪ ��1LŨ��ˮ���ɵõ��� Ʒ2������Ϊ g��
Ʒ2������Ϊ g��
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ �����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������____________________________________��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����22____��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c����Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2·6H2O��
d����MgCl2·6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ����__________________��Ŀ����_________________��
��������ȡþ�������У�Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��________________________________________________________________________��
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��Ϊʲô��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4����������������Ԫ�ء��䷴Ӧԭ��Ϊ____________________________________
________________________________________________________________________(��֬��HR����)��
�������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ________________________________________________________________________
________________________________________________________________________��
(4)��˾ƥ��(COOHOOCCH3) �ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
________________________________________________________________________��
�˷�Ӧ����������________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɻ�ͭ��(��Ҫ�ɷ���CuFeS2) ���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
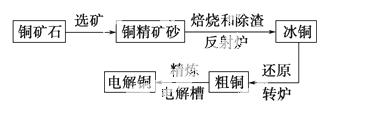
(1)�ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1 000 �����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ�����Ҳ���Fe������ת��Ϊ�ͼ�������ù�����������Ҫ��Ӧ�Ļ�ѧ����ʽ�ֱ���____________________��__________________________������¯������ ¯������Ҫ�ɷ���________��
¯������Ҫ�ɷ���________��
(2)��ͭ(Cu2S��FeS�����ۺ϶���)��Cu��Ϊ20%��50%��ת¯�У�����ͭ���ۼ�(ʯӢɰ)��1 200 �����Ҵ���������д�������ͭ�е�Cu2S��������Cu2O�����ɵ�Cu2O��Cu2S��Ӧ�����ɺ�Cu��ԼΪ98.5%�Ĵ�ͭ���ù��̷�����Ӧ�Ļ�ѧ����ʽ�ֱ���____________��
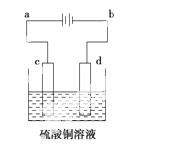
(3)��ͭ�ĵ�⾫������ͼ��ʾ���ڴ�ͭ�ĵ������У���ͭ��Ӧ��ͼ�е缫________(��ͼ�е���ĸ)���ڵ缫d�Ϸ����ĵ缫��ӦʽΪ____________________________������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�����ظ����ƣ�Na2Cr2O7���ᾧ���ĸҺ������������Fe3+�������ظ���أ�K2Cr2O7�����������̼���������ܽ��������ͼ
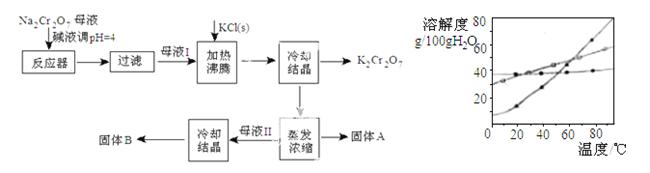 ��1����Na2Cr2O7����K2Cr2O7�Ļ�ѧ����ʽΪ
��1����Na2Cr2O7����K2Cr2O7�Ļ�ѧ����ʽΪ  ��ͨ������ᾧ��������K2Cr2O7��ԭ���� ��
��ͨ������ᾧ��������K2Cr2O7��ԭ���� ��
��2����Na2Cr2O7ĸҺ�мӼ�Һ��pH��Ŀ���� ��
��3������A��ҪΪ ���ѧ ʽ��
ʽ�� ������B��ҪΪ ���ѧʽ����
������B��ҪΪ ���ѧʽ����
��4������ˮϴ�ӹ���A�����յ�ϴ��Һת�Ƶ�ĸҺ ���I����II����III�����У�������߲����ֿ�ʹ�ܺ���͡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�����b��d��A2B���⻯���ΪV�η��ӣ�c�ģ�1�����ӱ�e�ģ�1��������8�����ӡ�
�ش��������⣺
(1)Ԫ��aΪ________��cΪ________��
(2)����ЩԪ���γɵ�˫ԭ�ӷ���Ϊ________��
(3)����ЩԪ���γɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε���________����ֱ���ε���________(д2��)��
(4)��ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ������________�����Ӿ������________�������������________�����Ӿ������________(ÿ����һ��)��
(5)Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͭ��һ������Ũ���ᷴӦ�õ�����ͭ��Һ��NO��N2O4��NO2 �Ļ�����壬��Щ������1.68��O2(��״��)��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�����������ᡣ���ͭ��Ӧ����������ʵ���������(����)
A��0.4mol B��0.55mol C��0.6mol D��0.65mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com