
����������Һ����˫��ˮ,ҽ������������ɱ��������������ϴ�˿ڡ��ش������й�˫��ˮ������:
(1)������Ӧ��,H2O2�����������Եķ�Ӧ����������(�����)��
A��Na2O2+2HCl 2NaCl+H2O2 2NaCl+H2O2 |
B��Ag2O+H2O2 2Ag+O2��+H2O 2Ag+O2��+H2O |
C��2H2O2 2H2O+O2�� 2H2O+O2�� |
D��3H2O2+Cr2(SO4)3+10KOH 2K2CrO4+3K2SO4+8H2O 2K2CrO4+3K2SO4+8H2O |
 A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������
A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������ 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣�
��1�������б仯�У��ٵ������ ���ռ��ۻ� ��MgCl2����ˮ ��HCl����ˮ,
δ������ѧ���ƻ����� �����������Ӽ��ƻ����� ������д��ţ�
��2���������������Т�Ne ��Na2O��NH3��KOH��ֻ���ڹ��ۼ�
���� ���ȴ��ڹ��ۼ��ִ������Ӽ����� ������д��ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��24��4 gNaOH��������ˮ���100 mL��Һ�����ܶ�Ϊ1��219 g/mL��
(1)����Һ��NaOH�����ʵ���Ũ��Ϊ ��
(2)����Һ��NaOH����������Ϊ ��
(3)�Ӹ���Һ��ȡ��10 mL������NaOH�����ʵ���Ũ��Ϊ ��NaOH����������Ϊ ����Һ���ܶ�Ϊ ����NaOH������Ϊ ����NaOH�����ʵ���Ϊ ��
(4)��ȡ����10 mL��Һ��ˮϡ�͵�100 mL��ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ⱦ�г����к���������Ŀ�����Ⱦ�⣬�ؽ�����������Һ�������ˮ����ȾҲ�൱���أ���������������ӵ�����ʽϿ��������ƣ�����β������Ҫ�к��ɷ�һ����̼�͵�����������˳��п�����Ⱦ���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ������о��ؽ�������ˮ��Ⱦ�Ĵ������зdz���Ҫ�����壮
��1��һ�������£�NO2��SO2��Ӧ����SO3��NO�������壮�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ�������������Ӧƽ��ʱNO2��SO2�����Ϊ1��6����ƽ�ⳣ��K�� ��
��2����ҵ�ϳ���Na2CO3��Һ���շ����������������NO��NO2�Ļ����Ϊ������
��֪��NO������Na2CO3��Һ��Ӧ��
NO��NO2��Na2CO3��2NaNO2��CO2��2NO2��Na2CO3��NaNO2��NaNO3��CO2
��������Na2CO3��Һ��ȫ����NO��NO2�Ļ���ÿ����22.4L����״����CO2��ȫ���ݳ���ʱ������Һ����������44g������������NO��NO2�������Ϊ ��
��3����ͼ��MCFCȼ�ϵ�أ�������ˮú����CO��H2��Ϊȼ�ϣ�һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ�AΪ��ص� ����ѡ�������������д��B���缫��Ӧʽ ��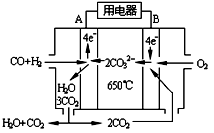
 ��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��֪��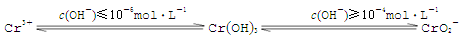
�ں���6�۸��ķ�ˮ�м���һ���������������������ʹ��6�۸���ԭ�ɣ�3�۸����ٵ�����ҺpH��6��8֮�䣬ʹFe3����Cr3��ת��ΪFe(OH)3��Cr(OH)3��������ȥ�������ӷ���ʽ��ʾ��ҺpH���ܳ���10��ԭ�� ��
��5������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�
CrO3����ǿ�����ԣ����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ����B��ʱʣ�����ijɷ��� ���ѧʽ����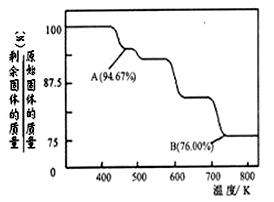
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��200 mg MnC2O4��2H2O���������������ȷֽ�ʱ�����ù�����������(m)���¶�(T)�仯������(��֪�����̲��ȶ�����������Ԫ�صĻ��ϼ���300 �����²���)��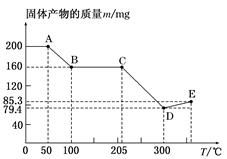
�Իش��������⣺
(1)д��B��������Ļ�ѧʽ��_________________________________________��
(2)��B�㵽C������й����������������ԭ����_____________________________________________________��
(3)ͨ������ȷ��D��������Է������������ƶ�������Ļ�ѧʽ��____________
(4)��D�㵽E������й��������������ӵ�ԭ����___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��200 mL ij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2 mol��L��1�����ᣬ����������������������������ϵ��ͼ��ʾ��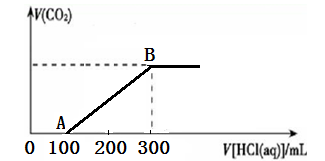
��1��OA�Ρ�AB�η�����Ӧ�����ӷ���ʽ__________________��__________________��
��2��B��ʱ����Ӧ������Һ�����ʵ����ʵ���Ũ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CO��O2��CO2�������9mL��������ָ���ԭ��״̬ʱ�������������1mL��ͨ��NaOH��Һ������ּ���5mL������������CO��O2��CO2����ȿ���Ϊ ___ __ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(N2H4)�ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�á���������������м��㣺
(1)�������������������ɵ�����һ���⻯����⻯�����Է�������Ϊ43.0�����е�ԭ�ӵ���������Ϊ0.977������ȷ�����⻯��ķ���ʽΪ________�����⻯����ײ������ȫ�ֽ�Ϊ������������4.30 g���⻯����ײ��������������ڱ�״���µ����Ϊ________L��
(2)��������������������������ƽ�����������ȼ�ϣ�����������������������Ӧ�����ǵ�����ˮ����������������������ɵĻ���ƽ�����ȫ��Ӧ����72.0 kgˮ�����ƽ���������������________��
(3)����ˮ��Һ����������NO��NO2������壬��Ӧ����ʽΪ6NO�� 4NH3=5N2��6H2O ��6NO2�� 8NH3=7N2��12H2O��NO��NO2�������180 mol��8.90��103g��ˮ(��������0.300)��ȫ���գ�����156 mol���������պ�ˮ�ܶ�Ϊ0.980 g/cm3����ٸû��������NO��NO2�������Ϊ________�������պ�ˮ�����ʵ���Ũ��________(�𰸱���1λС��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com