
| A������500mL1mol/Lϡ����ʵ���У�û��ϴ���ձ��Ͳ����� |
| B����������ᾧˮ�����ⶨʵ���У�����û���ڸ���������ȴ����ȴ������ƽ���� |
| C���ñ�����ζ�����NaOH��Һʵ���У�ʹ����ʽ�ζ��ܿ�ʼƽ�ӣ��������Ӷ��� |
| D�����к��Ȳⶨʵ���У���ϡ����������Һ��Ũ���ᷴӦ��õ��к�����ֵ |


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
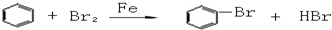
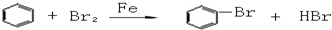
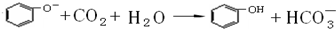
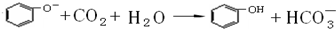
 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3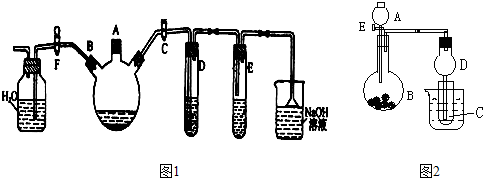
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1������ʵ��������ʵ����ʵ��������ȷ����
��1������ʵ��������ʵ����ʵ��������ȷ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿����ģ���ѧ�Ծ���B�������������� ���ͣ�ʵ����
[2012�������ʼ�]��11�֣�ij�о�ѧϰС��Ϊ̽��Fe3+�����Ƿ�������SO2����������µ�ʵ��װ�ã�����ʵ��������װ�õ����������ã���
��1����ͬѧ��������ʵ�߿���װ����ȡSO2������̽��ʵ�顣
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B�������� ��
�۷�Ӧһ��ʱ���ͬѧȡװ��C��������Һ�������м���HCl�ữ��BaCl2��Һ�۲쵽�а�ɫ�����������ɴ����ó����ۣ�Fe3+������SO2��
��2����ͬѧ��Ϊ��ͬѧ��ʵ�鲻�Ͻ��������������߿���װ�����װ��A����ʹװ��E���Լ���Ӧһ��ʱ��رջ���1������2���ַ�ӦƬ�̺�ȡװ��C��������Һ�������м�������KMnO4��Һ���۲쵽KMnO4��Һ�Ϻ�ɫ��ȥ���ɴ����ó����ۣ�Fe3+�ѱ�SO2��ԭ��Fe2+��
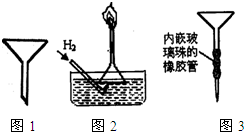
�ٸ�ʵ����H2ʱ����Ũ��������450mL3mol/L��ϡ���ᣬ����������IJ����������ձ�����Ͳ������������ͷ�ι��⣬���� ��
���ƹ�������������������û��ϴ���ձ��벣����������������Һ��Ũ�Ȼ� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
���ڸ�ʵ�������H2����������� ��
��3����ͬѧ��Ϊ�ס��ҵ�ʵ������Ͻ���Ϊ����������ͬѧ��ʵ��װ�ü�����������ʵ�顣��װ��F�ڷ�Ӧһ��ʱ���ȡװ��C��������Һ�������м���HCl�ữ��BaCl2��Һ���ó����ͬѧ��ͬ�Ľ��ۡ���������ش�
�ټ�ͬѧʵ�鲻�Ͻ���ԭ���� ����ѡ����ţ�
| A��SO2�ܽ���̫С |
| B��SO2����Fe3+������Ӧ |
| C��H2SO3��BaCl2����Ӧ |
| D��װ���еĿ�����SO2����ˮ��Ҳ������H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����в�ɽ�ظ�����ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
��15�֣�ijУ��ѧ��ȤС��Ϊ̽��SO2����Ļ�ԭ�����������װ��ͼ��

��1��SO2���廹ԭFe3+�����ӷ�Ӧ����ʽΪ ��
��2��ʵ���������SO2���������Ũ�����ͭ��Ӧ����ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ
���ڷ�Ӧ������H2SO4����Щ���� ��
��3��װ��C�������� ��
��4����Ҫ��A�еõ���Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ��������� ������ĸ��ţ���
A�������� B��ʯ���� C��©�� D���ձ�
E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ����ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ������죬��ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ����
����������������һ������ ����������ţ���ԭ���� ��
��6������װ�����ܱ���I-�Ļ�ԭ������SO2�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com