
��12�֣�����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������

��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ���AΪ ����д��ѧʽ����
д��B��C�ķ���ʽ ��
��2����AΪ��̬�����B��Cת������Ҫ��������AΪ ����д��ѧʽ����
д��A��B��ѧ����ʽ ��
д��Cu+D��Һ��B�����ӷ���ʽ ��
��3����ij��ɫ����Y���������ȣ�Ͷ�뵽����ij����ɫ��ҺD�в�������������ɵĻ������X����X��������ʾʵ�飺

д��M��G���������ʵĻ�ѧʽ M G
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������
��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ���AΪ ����д��ѧʽ����
д��B��C�ķ���ʽ ��
��2����AΪ��̬�����B��Cת������Ҫ��������AΪ ����д��ѧʽ����
д��A��B��ѧ����ʽ ��
д��Cu+D��Һ��B�����ӷ���ʽ ��
��3����ij��ɫ����Y���������ȣ�Ͷ�뵽����ij����ɫ��ҺD�в�������������ɵĻ������X����X��������ʾʵ�飺
д��M��G���������ʵĻ�ѧʽ M G
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������
��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ�
�� D�Ļ�ѧʽΪ���� ������
�� д��B��C�Ļ�ѧ����ʽ������������������������������������
��2����AΪ��̬�����aΪ���������ȣ�B��Cת������Ҫ������
��д��C��D��ѧ����ʽ����������������������������������������д��Cu��D��ϡ��Һ��Ӧ�����ӷ���ʽ��������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ��ƶ���
(18�֣�����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������
��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ�
�� D�Ļ�ѧʽΪ���� ������
�� д��B��C�Ļ�ѧ����ʽ������������������������������������
��2����AΪ��̬�����aΪ���������ȣ�B��Cת������Ҫ������
�� A�Ļ�ѧʽΪ���� ��������1mol A�к��еĵ�����Ϊ���� ������NA (NA Ϊ�����ӵ���������
�� д��C��D��ѧ����ʽ���������������������������������������÷�Ӧ���������뻹ԭ��������֮��Ϊ�������������� ��
?д��Cu��D��Һ��Ӧ�����ӷ���ʽ��������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
(18�֣�����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������
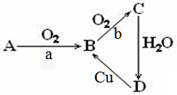
��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ�
�� D�Ļ�ѧʽΪ���� ������
�� д��B��C�Ļ�ѧ����ʽ������������������������������������
��2����AΪ��̬�����aΪ���������ȣ�B��Cת������Ҫ������
�� A�Ļ�ѧʽΪ���� ��������1mol A�к��еĵ�����Ϊ���� ������NA (NA Ϊ�����ӵ���������
�� д��C��D��ѧ����ʽ���������������������������������������÷�Ӧ���������뻹ԭ��������֮��Ϊ�������������� ��
?д��Cu��D��Һ��Ӧ�����ӷ���ʽ��������������������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com