
��08���Ϻ�������֪��H2(g)��F2(g)=2HF(g)��270kJ������˵����ȷ���ǣ� ��
A��2L����������ֽ��1L��������1L�ķ�������270kJ����
B��1mol������1mol������Ӧ����2molҺ̬������ų�������С��270kJ
C������ͬ�����£�1mol������1mol�����������ܺʹ���2mol���������������
D��1������������1���������ӷ�Ӧ����2����������ӷų�270kJ
����C
��������ѧ����D�����Ȼ�ѧ����ʽ�ĺ��塣
�����������ϵĻ�ѧ����ʽ��֪��1mol������1mol������Ӧ����2mol��̬������ų��������ų�������Ϊ270kJ����������ķ�����ת��ΪҺ̬�ķ����Ҫ�ų���������������Ӧ����270kJ�����ڷ�ӦΪ���ȷ�Ӧ����������ͬ�����£�1mol������1mol�����������ܺʹ���2mol�����������������
���֪ʶ����ɣ�
����Ȼ�ѧ����ʽ���ж�������
�١���������̼��ȫȼ�����ɶ�����̼ʱ�ų��������Ȳ���ȫȼ������һ����̼ʱ�ų��������࣬��HԽС��
�ڡ�����������̬������ȫȼ�����ɶ�������ʱ�ų��������ȹ�̬������ȫȼ�����ɶ�������ʱ�ų��������࣬��HԽС��
�ۡ���������������ȫȼ������Һ̬ˮʱ�ų�����������ȫȼ��������̬ˮʱ�ų��������࣬��HԽС��
�ܡ���������̬ˮ������Һ̬ˮ��ǰ���£�ͬһ����ȼ�ϣ�N2H4 ��CH4 ��C3H8����ȫȼ�գ�ȼ�ϵ�����Խ�ų�������Խ�࣬��HԽС��
�ݡ���������̬ˮ������Һ̬ˮ��ǰ���£�ͬһ����ȼ�ϣ�N2H4 ��CH4 ��C3H8����ȫȼ�յıȲ���ȫȼ�յķų��������࣬��HԽС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
1 | 10mLFeSO4��Һ | 10mLNH3 | ���ɰ�ɫ���������ɫ |
2 | 20mLH2S | 10mLSO2 |
|
3 | 30mLNO2(��Ҫ) | 10mLH2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
4 | 15molCl2 | 40mLNH3 |
|
(1��ʵ��1�У��������ձ�Ϊ________ɫ��д��������ɫ�Ļ�ѧ����ʽ________________________��
(2��ʵ��2����Ͳ�ڵ������ǣ���___________���ɣ�����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
(3��ʵ��3�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ_______________________________��
(4��ʵ��4�У���֪��3Cl2��2NH3��N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08���Ϻ�������֪�� ����A����������ø�������»�����Ϊ�к�����GHB����ͼ�ǹ�������A��һ���Ʊ���������A������һϵ�л�ѧ��Ӧ��
![]()
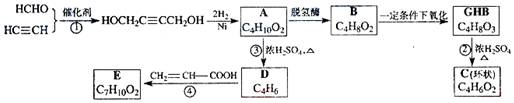
��ش��������⣺
(1��д����Ӧ���ͣ���Ӧ��____________����Ӧ��____________��
(2��д��������B�Ľṹ��ʽ_____________________________��
(3��д����Ӧ�ڵĻ�ѧ����ʽ____________________________��
(4��д����Ӧ�ܵĻ�ѧ����ʽ____________________________��
(5����Ӧ���г�����E�⣬�����ܴ���һ�ָ�����(��![]() �ṹ)�����Ľṹ��ʽΪ________________��
�ṹ)�����Ľṹ��ʽΪ________________��
(6���뻯����E��Ϊͬ���칹������ʲ�����Ϊ________����д��ĸ����
a���� b��ȩ c������ d����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08���Ϻ�������֪���ȵļ�����Һ�У�NaClO�������·�Ӧ��3NaClO![]() 2NaCl��NaClO3������ͬ������NaClO2Ҳ�ܷ������Ƶķ�Ӧ�������ղ����ǣ� ��
2NaCl��NaClO3������ͬ������NaClO2Ҳ�ܷ������Ƶķ�Ӧ�������ղ����ǣ� ��
A��NaCl��NaClO B��NaCl��NaClO3 C��NaClO��NaClO3 D��NaClO3��NaClO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08���Ϻ�����![]() ��֪�����Ħ������ԽС����ɢ�ٶ�Խ�졣��ͼ��ʾΪ������ɢ�ٶȵ�ʵ�飬����������ɢ����ʱ�γɰ�ɫ�̻������й��ڼס��ҵ��ж���ȷ���� ����
��֪�����Ħ������ԽС����ɢ�ٶ�Խ�졣��ͼ��ʾΪ������ɢ�ٶȵ�ʵ�飬����������ɢ����ʱ�γɰ�ɫ�̻������й��ڼס��ҵ��ж���ȷ���� ����
A������Ũ��ˮ������Ũ���� B������Ũ���ᣬ����Ũ��ˮ
C������Ũ��ˮ������Ũ���� D������Ũ���ᣬ����Ũ��ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com