
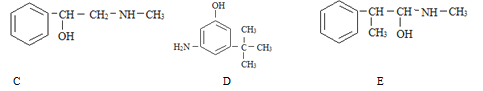
����Ŀ��(1)��A������ B������ C���Ҵ� D�������ʵ������л����У�ѡ����ʵ����ʣ����������ں����ϡ�
����˿����Ҫ�ɷ���__________��
���ҹ���������������������Ҫ�ɷ���____________��
���Ƽ���ָ��ʻԱ������������____________�������ꡣ
���������ˮƿ�ڵ�ˮ��[��Ҫ�ɷ�CaCO3��Mg(OH)2]����____________��
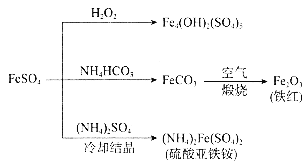
(2)A��B��C��D��E��Ϊ��ѧ�����л����ת����ϵ����ͼ���ش��������⣺
![]()
��E���ʵĽṹ��ʽΪ____________��AB�Ļ�ѧ����ʽΪ____________��
��ʵ��������A��C��ȡD����Ӧ�Ļ�ѧ����ʽΪ_____���ռ�װ�ÿ�ѡ����ͼ______װ��(����)��
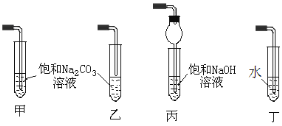
���𰸡�D A C B ![]() 2C2H5OH+O2
2C2H5OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ��
CH3COOCH2CH3+H2O ��
��������
(1) ����˿����Ҫ�ɷ��ǵ����ʣ����ҹ���������������������Ҫ�ɷ��Ǽ��飻���Ƽ���ָ��ʻԱ�����������оƾ����Ҵ��ĺ������ꣻ���������ˮƿ�ڵ�ˮ��[��Ҫ�ɷ�CaCO3��Mg(OH)2]�������ᣬ�ʴ�Ϊ��D��A��C��B��
(2)��ϩ��ˮ�����ӳɷ�Ӧ�õ��Ҵ����Ҵ������õ���ȩ����ȩ�������õ����ᣬ�Ҵ������� ����������Ӧ�õ�������������ϩ�����Ӿ۷�Ӧ�õ�����ϩ��
��EΪ����ϩ���ṹ��ʽΪ��![]() ��AB���Ҵ�����������ȩ�Ĺ��̣���ӦΪ��2C2H5OH+O2
��AB���Ҵ�����������ȩ�Ĺ��̣���ӦΪ��2C2H5OH+O2![]() 2CH3CHO+2H2O���ʴ�Ϊ��
2CH3CHO+2H2O���ʴ�Ϊ��![]() ��2C2H5OH+O2
��2C2H5OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��ʵ��������A��C��ȡD�����Ҵ������ᷢ��������Ӧ�õ�������������ӦΪ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���ռ�װ��Ӧѡ���ң�����̼������Һ�����ܽ��Ҵ����к����ᣬ���������������ܽ�ȱ�����������������Һ���Ͽ��Է���ֹ������ѡ�ң���װ�õ���������Һ�л����������ʲ�ѡ����װ���������ƻᵼ����������ˮ�⣬�ʲ�ѡ����װ��ˮ������Ч��ȥ�Ҵ������ᣬ�ʲ�ѡ���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���ռ�װ��Ӧѡ���ң�����̼������Һ�����ܽ��Ҵ����к����ᣬ���������������ܽ�ȱ�����������������Һ���Ͽ��Է���ֹ������ѡ�ң���װ�õ���������Һ�л����������ʲ�ѡ����װ���������ƻᵼ����������ˮ�⣬�ʲ�ѡ����װ��ˮ������Ч��ȥ�Ҵ������ᣬ�ʲ�ѡ���ʴ�Ϊ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���ң�
CH3COOCH2CH3+H2O���ң�


 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п��п�Ļ�����Ӧ�ù㷺�����磬�ⶨͭ�Ͻ��е�Ǧ��пʱҪ����п�����ӵ����з�Ӧ��
[Zn(CN)4]2-��4HCHO��4H2O==Zn2+��4HOCH2CN��4OH�����ش��������⣺
(1)��̬Zn2+�ĵ����Ų�ʽΪ_____________����̬ Cԭ�Ӻ������ռ��_____����ͬԭ�ӹ����
(2)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��HOCH2CN�����к��е�������������Ŀ֮��Ϊ_________��
(3)HCHO������̼ԭ�ӹ�����ӻ�������________������������HCHO��ˮ��Һ��HCHO������ˮ���ܵ���Ҫԭ����_________________________��
(4)[Zn(CN)4]2-��Zn2+��CN��֮��Ļ�ѧ����Ϊ_________���ṩ�µ��ӶԵijɼ�ԭ����________��
(5)Zn��S�γ�ij�ֻ�����ľ�����ͼ��ʾ��
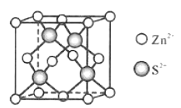
��Zn2������S2����ɵ�___________��϶�У�
����֪�����ܶ�Ϊd g/cm3���þ����ı߳�����ʽΪ______pm(д�������ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CoC2O4���Ʊ������ܵ�ԭ�ϡ����ú��ܷ���(��Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��CaO��MgO��̼���л����)��ȡCoC2O4�Ĺ���������ͼ��
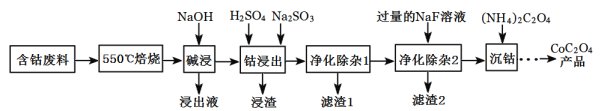
(1)��550����������Ŀ����_______��
(2)������Һ������Ҫ�ɷ���_______��
(3)���ܽ�����������Co3+ת��ΪCo2+����Ӧ�����ӷ���ʽΪ_________��
(4)����������1�������У�����40��50������H2O2��Һ����Ŀ����______��(�����ӷ���ʽ��ʾ)����������80��85��������Na2CO3��Һ����pH��5��������I������Ҫ�ɷ���______��
(5)����������2���ɽ��ơ�þ����ת��Ϊ�������˳�ȥ����������Һ��c(Ca2+)=1.0��l0-5mol/L������Һ��c(Mg2+)Ϊ___[��֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10]
(6)Ϊ�ⶨ�Ƶõ�CoC2O4��Ʒ�Ĵ��ȣ��ֳ�ȡ1.000g��Ʒ���������ʵ��Լ�ת��Ϊ�����[(NH4)2C2O4]��Һ�����ù���ϡ�����ữ����0.1000mol/L���������Һ�ζ����ﵽ�ζ��յ㣬����ȥ���������Һ26.00mL���ò�Ʒ�Ĵ���Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ�������й�������ȷ���ǣ� ��
A. 2 L 0.5 mol��L-1CH3COOH��Һ�У�CH3COO- ����ĿΪNA
B. һ��������ij���ʣ�����������Ϊn NA����������һ��С��nNA
C. ��״���£�22.4LHF���еĹ��ۼ���ΪNA
D. ��FeI2��Һ��ͨ��һ����Cl2����1molFe2+������ʱ��ת�Ƶĵ�������С��3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ֳƻƼ���ҹ��ض�����ҩ������������е�һ���������ҹ���ѧ���о�������ṹ����ͼ�����и�����������ػ�Ϊͬ���칹�����_____(����ĸ����ͬ)����Ϊͬϵ�����_____��
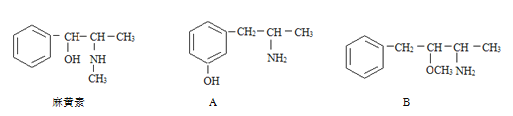
��2�����ȡ����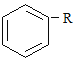 ���Ա�KMnO4��������Һ��������
���Ա�KMnO4��������Һ��������  ���������R��ֱ
���������R��ֱ
���뱽�����ӵ�̼ԭ����û��CһH���������ױ������õ� �� ���з���ʽ��C11H16��һ���ȡ��������֪�����Ա�������Ϊ���칹�干��7�֣����е�3����
�� ���з���ʽ��C11H16��һ���ȡ��������֪�����Ա�������Ϊ���칹�干��7�֣����е�3����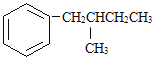
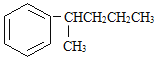
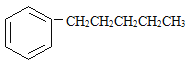
д����4�ֵĽṹ��ʽ��
1________ | 2________ |
3________ | 4________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڼס��ҡ���������ͬ�ܱ������а���ͬ��ʽͶ�ϣ�һ�������·�����Ӧ(��ʼ�¶Ⱥ���ʼ�����ͬ):N2(g)+3H2(g)![]() 2NH3(g) ��H<0������������±���ʾ:
2NH3(g) ��H<0������������±���ʾ:
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | lmolN2��3molH2 | 2molNH3 | 2molNH3 |
ƽ��ʱ������� | V�� | V�� | V�� |
��Ӧ��ƽ�ⳣ��K | K�� | K�� | K�� |
ƽ��ʱNH3��Ũ��/mol/L | c�� | c�� | c�� |
ƽ��ʱNH3�ķ�Ӧ����/mol/(L��min) | v�� | v�� | v�� |
����˵����ȷ����
A. V��>V�� B. K��>K�� C. c��>c�� D. V��=V��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I2��KI��Һ�д���ƽ�⣺I2(aq)+I-(aq)![]() I3-(aq)��ijI2��KI�����Һ�У�c(I3-)���¶�T��ƽ������ͼ��ͼ������˵������ȷ����
I3-(aq)��ijI2��KI�����Һ�У�c(I3-)���¶�T��ƽ������ͼ��ͼ������˵������ȷ����
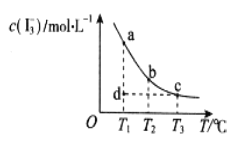
A.��ӦI2(aq)+I-(aq)![]() I3-(aq)����H>0
I3-(aq)����H>0
B.���¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1>K2
C.����Ӧ���е�״̬dʱ��һ����![]() ��>
��>![]() ��
��
D.״̬a��״̬b��ȣ�״̬a��c(I2)С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������;�㷺�������Ʊ���ͼ��ʾ���ʣ�����˵���������( )
A.![]() ���ڼ�ʽ�Σ���������ˮ��
���ڼ�ʽ�Σ���������ˮ��
B.������![]() ��Ӧ�����ӷ���ʽΪ��
��Ӧ�����ӷ���ʽΪ��![]()
C.����ϡ�����![]() ��Һ�����������Ƿ���
��Һ�����������Ƿ���![]()
D.����ȴ�ᾧ����IJ����ǹ��ˣ�����������������Ҫ�����оƾ��ơ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ƕ������н�������ǿ��Ԫ�أ�F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С������Ԫ�ء���ش��������⣺
��1��C��Ԫ�����ڱ��е�λ��Ϊ___��G��ԭ�ӽṹʾ��ͼ��___��
��2��D��E��ԭ�Ӹ�����1��1�γɻ�����ף������ʽΪ___��������ѧ������Ϊ___������еμ�����ˮʱ������Ӧ�Ļ�ѧ����ʽ��___��
��3��E��F��G����Ԫ�����γɵļ����ӣ��뾶�ɴ�С��˳����___��(�����ӷ��ű�ʾ)
��4����BA4��D2��EDA��ˮ��Һ���ȼ�ϵ�أ��缫����Ϊ����Խ����缫����a��ͨ��BA4���壬b��ͨ��D2���壬��a���Ǹõ�ص�___���������ĵ缫��ӦʽΪ___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com