
����������ȼ�գ������������ֹ�̬������(P2O3��P2O5)��3.1 g�ĵ�����(P)��3.2 g������ȼ�գ�����Ӧ��ľ����ų�x kJ������
(1)ͨ������ȷ����Ӧ��������(�û�ѧʽ��ʾ)��_________��
����Ӧ������(g)�ֱ�Ϊ______________��
(2)��֪������ȼ����Ϊy kJ·mol��1����ʾ������ȼ�յ��Ȼ�ѧ����ʽΪ______________________________��
(3)����P2O3�������з�Ӧ���ɹ���P2O5���Ȼ�ѧ����ʽΪ________________________________________��
�𰸡�(1)P2O3��P2O5��2.75 g��3.55 g
(2)P(s)��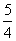 O2(g)===
O2(g)=== P2O5(s)��
P2O5(s)��
��H����y kJ·mol��1��
P(s)��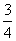 O2(g)===
O2(g)=== P2O3(s)��
P2O3(s)��
��H����(20x��y)kJ·mol��1��
(3)P2O3(s)��O2(g)===P2O5(s)��
��H��(40x��4y)kJ·mol��1
�������跴Ӧ������P2O3��P2O5�����ʵ����ֱ�Ϊm��n��

��
���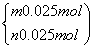
�ʿɷֱ����P2O5��P2O3��������
(2)������֪ȼ���ȼ�Ϊ1mol������P2O5ʱ�ķ�Ӧ�ȣ���P(s)��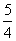 O2(g)===
O2(g)=== P2O5(s)����H����y kJ·mol��1��������0.025mol P2O5�ų�������Ϊ
P2O5(s)����H����y kJ·mol��1��������0.025mol P2O5�ų�������Ϊ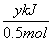 ��0.025mol��0.05y kJ��
��0.025mol��0.05y kJ��
1mol��������O2��Ӧ���ɹ���P2O3�ķ�Ӧ�ȣ�
��H��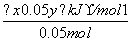 ��1mol����(20x��y)kJ·mol��1
��1mol����(20x��y)kJ·mol��1
(3)P(s)��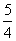 O2(g)===
O2(g)=== P2O5(s)��
P2O5(s)��
��H����y kJ·mol��1��
P(s)��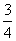 O2(g)===
O2(g)=== P2O3(s)��
P2O3(s)��
��H����(20x��y)kJ·mol��1��
���ݸ�˹���ɢ١�2���ڡ�2����P2O3(s)��O2(g)===P2O5(s)
��H��(40x��4y)kJ·mol��1��


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������и��鷴Ӧ����Ӧ��ʼʱ����H2����������(����)
A��70 �棬��0.1 mol Mg�ۼ��뵽10 mL 6 mol·L��1��HNO3��Һ��
B��60 �棬��0.1 mol Mg�ۼ��뵽10 mL 3 mol·L��1��������
C��60 �棬��0.1 mol Fe�ۼ��뵽10 mL 3 mol·L��1��������
D��60 �棬��0.1 mol Mg�ۼ��뵽10 mL 3 mol·L��1��H2SO4��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��M��������Ԫ�أ�����X��Z�õ������ӷ���ϡ������ʱ��X�ܽ⣬Z�����������ų��������Y2����Z2���������Һʱ��Y����������֪M2����������ǿ��Y2�����������ֽ����Ļ����ǿ������˳��Ϊ(����)
A��X>Z>Y>M B��X>Y>Z>M
C��M>Z>X>Y D��X>Z>M>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦA��B����C(��H<0)���������У���A��B����X(��H>0)����X����C(��H<0)��
����ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����(����)
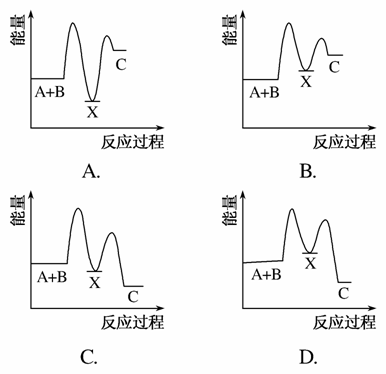
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ƾ���Ϊ����ȼ�ϵļ����Ѿ�����ȫ���ƹ㡣��֪C8H18��C2H5OHȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
2C8H18(l)��25O2(g)===16CO2(g)��18H2O(l)����H����10900 kJ·mol��1
C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)�� ��H����1367 kJ·mol��1
�ٶ����͵ijɷ�ΪC8H18���������Ӿƾ�������������ȼ��ʱ�����ܴﵽ��Ŀ����(����)
A����ʡ��ʯȼ��
B�������к�������ŷ�
C��������ת����ʣ����ʳ
D�����ÿǧ��ȼ��ȼ�շų�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л��������˵������ȷ����(����)
A������������鶼�DZ�����
B���������ϩ��������������Ӧ
C�����ͼױ����ɱ�KMnO4������Һ����
D������ϩ�������������ӳɷ�Ӧ���屽���������������ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������������̬����ɡ�2.24 L�û��������ȫȼ�պõ�4.48 L������̼(����������Ϊ��״��)��3.6 gˮ�������������������(����)
A��CH4��C3H8 B��CH4��C3H4
C��C2H6��C3H4 D��C2H4��C2H6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ����ȩ����ȩ��ɵĻ�����У���Ԫ�ص�����������9%������Ԫ�ص�����������(����)
A��16% B��37%
C��48% D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��л����˵������ȷ����(����)
A�����Ǻ�̼Ԫ�صĻ����ﶼ�����л���
B�����������͡��ƾ��������л��ܼ��е�����һ�����л���
C�����е��л��ﶼ������ȼ��
D���л��ﲻһ����������ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com