
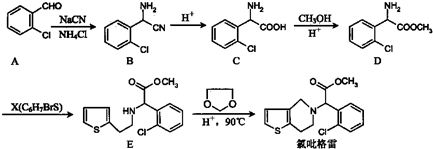
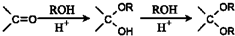
 �ĺϳ�·������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ�����Լ���ѡ����
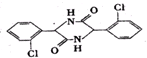 ��
�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ���÷�Ӧ����ʽΪ
���÷�Ӧ����ʽΪ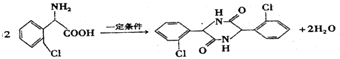 ��
�� ��
��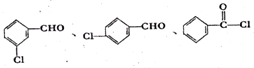 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ���ϳ�·������ͼΪ��
���ϳ�·������ͼΪ��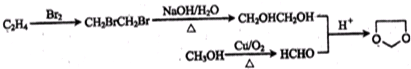 ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����³�ѹ�£�14 g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA |
| B��78 g������C=C˫������ĿΪ3 NA |
| C��1 L 1 mol?L-1��NaClO��Һ�к���ClO-����ĿΪNA |
| D����״���£�6.72 L NO2��ˮ��ַ�Ӧת�Ƶĵ�����ĿΪ0.1 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
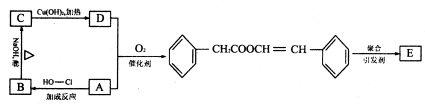
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol?L-1HCl����Һ�����к͵ζ����÷�̪��ָʾ������
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol?L-1HCl����Һ�����к͵ζ����÷�̪��ָʾ������| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol?L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.11 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 0.22 | 26.31 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | ��������� mL �� | ��NaOH��Һ�������mL�� |
| 1 | 20.00 | 18.20 |
| 2 | 17.10 | |
| 3 | 16.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
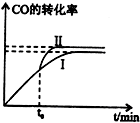 ��������һ����Ҫ�����ȼ�ϣ��ж��ֺϳɷ�����
��������һ����Ҫ�����ȼ�ϣ��ж��ֺϳɷ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A���⻯淋ĵ���ʽ�� |
B������ױ��Ľṹ��ʽ�� |
| C��������Ϊ146��������Ϊ92���ˣ�U��ԭ�ӣ�23892U |
D����ԭ�ӵĽṹʾ��ͼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ ����F
����F�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com